Are you looking for an answer to the topic “When an atom of chlorine forms an ion the atom?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Chlorine gains an electron, leaving it with 17 protons and 18 electrons. Since it has 1 more electron than protons, chlorine has a charge of −1, making it a negative ion. When ions form, atoms gain or lose electrons until their outer energy level is full.Hint: Chlorine is a highly negative ion, if the number of atoms it leads in the increase of radius and ultimately the repulsion increases too.Cl atomic number is 17 (17 electrons, 17 protons), when it gains electrons then Cl atom becomes Cl- which consists of 18 electrons and 17 protons.Most of the elements that make ionic compounds form an ion that has a characteristic charge. For example, sodium makes ionic compounds in which the sodium ion always has a 1+ charge. Chlorine makes ionic compounds in which the chloride ion always has a 1− charge.
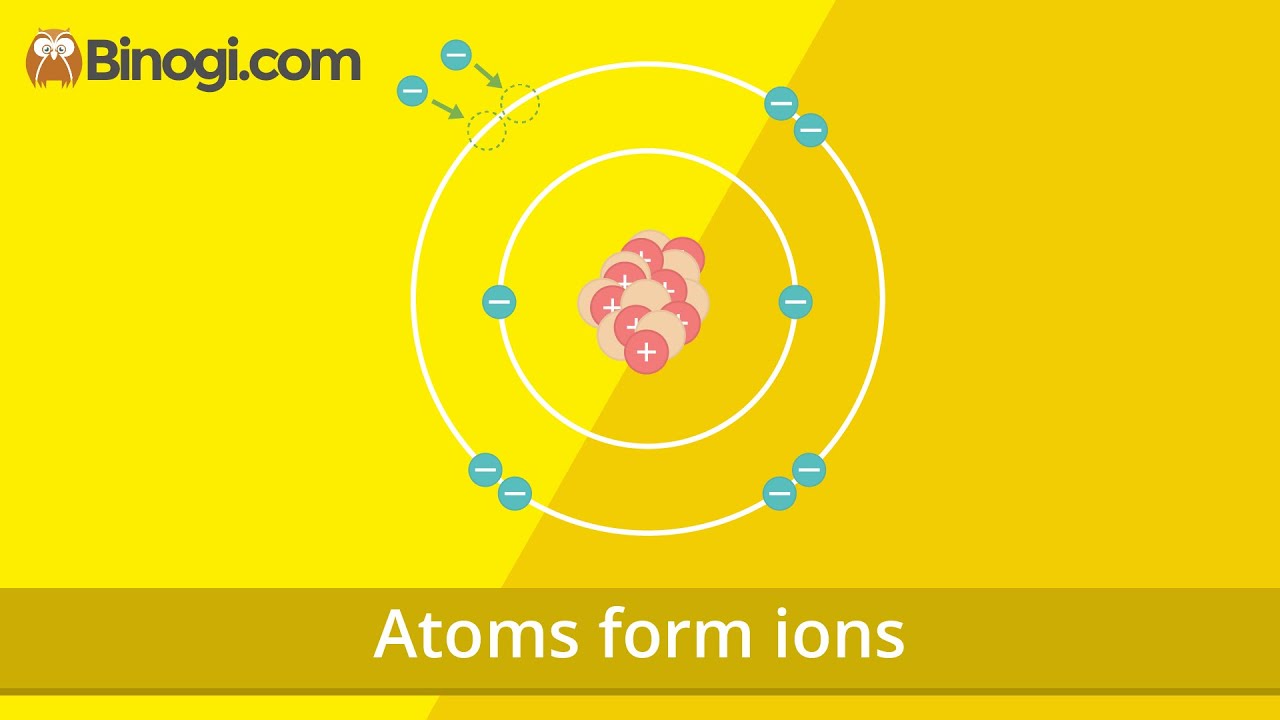
When a chlorine atom becomes a chloride ion the?
Hint: Chlorine is a highly negative ion, if the number of atoms it leads in the increase of radius and ultimately the repulsion increases too.Cl atomic number is 17 (17 electrons, 17 protons), when it gains electrons then Cl atom becomes Cl- which consists of 18 electrons and 17 protons.
When a chlorine atom forms an ion what charge does it have?
Most of the elements that make ionic compounds form an ion that has a characteristic charge. For example, sodium makes ionic compounds in which the sodium ion always has a 1+ charge. Chlorine makes ionic compounds in which the chloride ion always has a 1− charge.
Atoms form ions (Chemistry) – Binogi
Images related to the topicAtoms form ions (Chemistry) – Binogi

When an atom of chlorine forms an ionic bond?
When an atom of chlorine forms an ionic bond with an atom of sodium, the atom of chlorine gains a electron to form an anion. Explanation: An ionic bond is formed when an element completely transfers its valence electron to another element.
What happens when chlorine forms an ion?
Chlorine gains an electron, leaving it with 17 protons and 18 electrons. Since it has 1 more electron than protons, chlorine has a charge of −1, making it a negative ion. When ions form, atoms gain or lose electrons until their outer energy level is full.
What ion is formed with chlorine?
The chloride ion /ˈklɔːraɪd/ is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.
Is chlorine a cation or anion?
…
Cation vs anion chart.
| Cation | Anion | |
|---|---|---|
| Examples | Sodium (Na+), Iron (Fe2+), Ammonium (NH4+) | Chloride (Cl–), Bromide (Br–), Sulfate (SO42–) |
Why do chloride ions have a 1 charge?
this atom needs one more electron to form an ion with a full outer shell. Hence the ion formed will have a negative charge (adding electrons is adding a negative charge). As there is only one electron being added to the outer shell, the chloride ion formed will have a charge of 1- (and the ion symbol will be Cl–).
See some more details on the topic When an atom of chlorine forms an ion the atom? here:
When an atom of chlorine forms an ionic bond with … – Socratic
The atom of chlorine forms an anion with a charge of -1. The sodium atom loses its one valence electron to form a cation with a charge of +1 …
When an atom of chlorine forms an ionic bond with … – Brainly.in
Chlorine gains an electron, leaving it with 17 protons and 18 electrons. Since it has 1 more electron than protons, chlorine has a charge of …
What happens when an atom of chlorine forms a chlorine ion?
When a chlorine atom becomes a chloride ion, it is reduced. This means that the chlorine atom gains an electron and attains a negative charge.
Atoms vs. Ions
Atoms that gain extra electrons become negatively charged. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. By adding one more …
When a chlorine atom forms an ion its radius increases?
Answer: When a chlorine atom forms an ion, it gains electrons, making it negative. A neutral chlorine will become a chlorine with a -1 charge. When it gains an electron, the radius increases.
Why does chlorine gain an electron?
Chlorine (Cl) in its lowest energy state (called the ground state) has seven electrons in its outer shell. Again, it is more energy-efficient for chlorine to gain one electron than to lose seven.
Which atoms form an ionic bond?
Ionic bonds form only between metals and nonmetals. That’s because metals “want” to give up electrons, and nonmetals “want” to gain electrons. It takes energy to remove valence electrons from an atom and form a positive ion.
GCSE Chemistry – Formation of Ions #13
Images related to the topicGCSE Chemistry – Formation of Ions #13
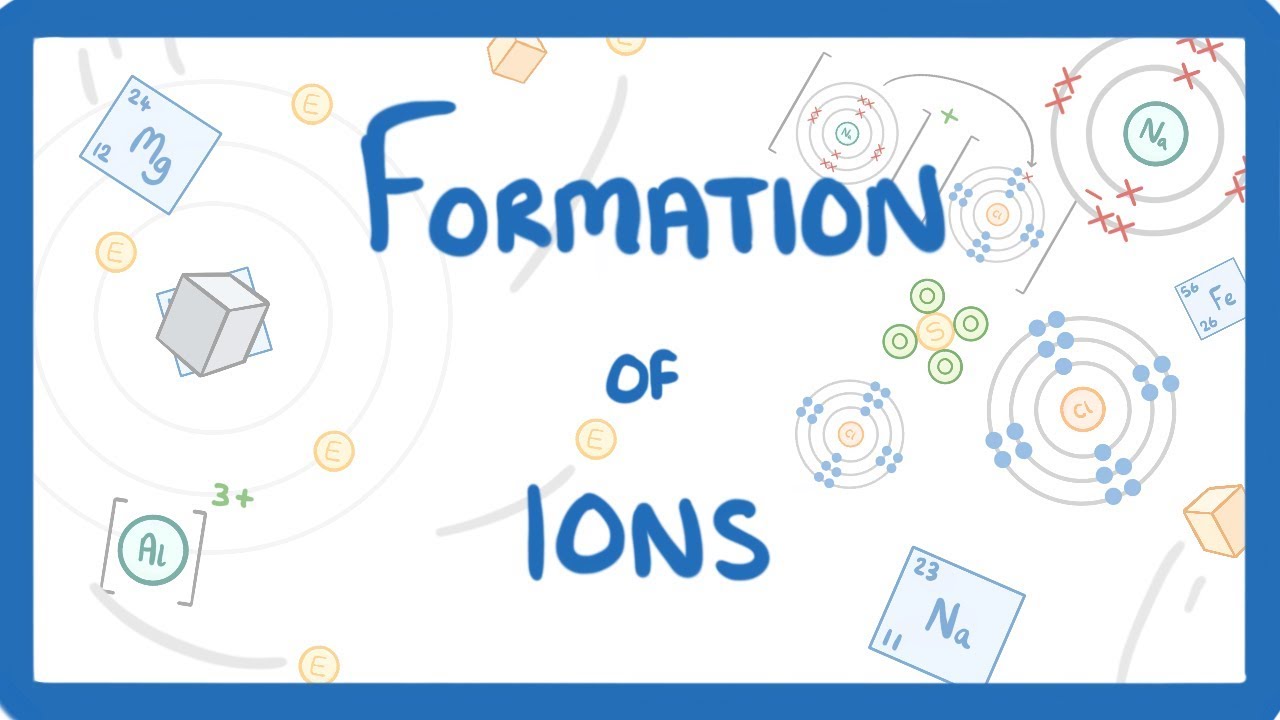
When a chlorine atom gains one more electron to become stable What type of ion will it form?
Chlorine also has ten core electrons and valence electrons in the third energy level. However, chlorine has seven valence electrons, one less than the noble gas argon, which has eight valence electrons. Thus, chlorine will gain one electron, forming the anion, Cl–, and achieving a stable noble gas configuration.
Why do atoms lose or gain electrons?
Atoms and chemical species lose or gain electrons when they react in order to gain stability. Thus, typically, metals (with nearly empty outer shells) lose electrons to non-metals, thereby forming positive ions. The number of electrons depends on their position on the Periodic table (in simple terms).
How many electrons will chlorine gain or lose?
Chlorine has 7 electrons in its valence shell. To meet the octet rule, it must either gain one electron or lose seven electrons. Gaining one is easier than losing seven so it will gain one electron to have a total of eight electrons when it forms an ion (i.e. charged particle).
What is the symbol of the ion formed when a chlorine atom gains one electron?
Similarly, if a chlorine atom gains an extra electron, it becomes the chloride ion, Cl–. Both ions form because the ion is more stable than the atom due to the octet rule.
How do you find the ion of chlorine?
The test for chloride ions described here is based on precipitation of an insoluble chloride salt. When a few drops of a silver nitrate solution are added to a slightly acidic aqueous solution that contains chloride ions, a white precipitate of silver chloride will form.
How many electrons are in a chlorine ion?
2: The Formation of a Chlorine Ion. On the left, the chlorine atom has 17 electrons. On the right, the chloride ion has 18 electrons and has a 1− charge.
Which atom is most likely to form a 3+ ion?
Thus, a nitrogen atom will form an anion with three more electrons than protons and a charge of 3−. The symbol for the ion is N3−, and it is called a nitride ion.
Is chloride A ion?
Chloride Ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride salts, and is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating fluid in and out of cells.
Cl- Electron Configuration (Chloride Ion)
Images related to the topicCl- Electron Configuration (Chloride Ion)

Does chlorine form a cation?
Chlorine cation | Cl+ – PubChem.
How does an atom become an ion?
Ions are formed by the addition of electrons to, or the removal of electrons from, neutral atoms or molecules or other ions; by combination of ions with other particles; or by rupture of a covalent bond between two atoms in such a way that both of the electrons of the bond are left in association with one of the …
Related searches to When an atom of chlorine forms an ion the atom?
- what is the ionic charge of an atom of sodium when it forms an ionic bond
- when an atom of chlorine forms an ionic bond with an atom of sodium the atom of chlorine
- what is the ionic charge of an atom of each element when it forms an ionic bond nitrogen
- when an atom of chlorine forms an ionic bond
- atoms form ions to
- when a chlorine atom forms an ion its radius increases
- what type of ion will chlorine tend to form
- which ion is an oxyanion
- which ion is an oxyanion?
- what is the ionic charge of an atom of nitrogen when it forms an ionic bond
- what occurs when an atom of chlorine and an atom of hydrogen
Information related to the topic When an atom of chlorine forms an ion the atom?
Here are the search results of the thread When an atom of chlorine forms an ion the atom? from Bing. You can read more if you want.
You have just come across an article on the topic When an atom of chlorine forms an ion the atom?. If you found this article useful, please share it. Thank you very much.