Are you looking for an answer to the topic “When an exchange of energy happens with the surroundings and the system but matter is not transferred from or to the system this type of system is?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
An (ideally) insulated system is an isolated system where neither matter nor energy can be transferred between the system and surroundings while a closed system is one where matter cannot be transferred but energy (heat) can be transferred to/from the surroundings.In thermodynamics, a closed system can exchange energy (as heat or work) but not matter, with its surroundings.(II) Closed system: A system which can exchange energy but not matter with its surroundings is called a closed system.

What is a system called when energy is exchanged between the system and the surroundings but matter is not exchanged?
In thermodynamics, a closed system can exchange energy (as heat or work) but not matter, with its surroundings.
Is a system can exchange energy but not matter with its surroundings?
(II) Closed system: A system which can exchange energy but not matter with its surroundings is called a closed system.
First Law of Thermodynamics, Basic Introduction – Internal Energy, Heat and Work – Chemistry
Images related to the topicFirst Law of Thermodynamics, Basic Introduction – Internal Energy, Heat and Work – Chemistry

Which type of system allows only energy to be exchanged with the surroundings?
Closed: this is a system in which only energy is being exchanged with the surroundings.
What is a system called when neither energy nor matter is exchanged between the system and the surroundings closed system free energy isolated system open system?
A system which can neither exchange matter nor energy with the surroundings called as isolated system. Since the boundary is sealed and isolated, there is no interaction between system and surrounding. Ice in an ideal thermos flask represents an isolated system.
What process transfers thermal energy from the surroundings to the system?
Thermal energy transfers occur in three ways: through conduction, convection, and radiation. When thermal energy is transferred between neighboring molecules that are in contact with one another, this is called conduction.
What is system and surrounding in thermodynamics?
A system of thermodynamics can be defined as a matter or region on which analysis is done. The system is separated from surrounding by the boundary. Everything external to the system is surrounding. System and surroundings in thermodynamics together is called a universe.
What is isolated system in thermodynamics?
In physical science, an isolated system is either of the following: a physical system so far removed from other systems that it does not interact with them. a thermodynamic system enclosed by rigid immovable walls through which neither mass nor energy can pass.
See some more details on the topic When an exchange of energy happens with the surroundings and the system but matter is not transferred from or to the system this type of system is? here:
The laws of thermodynamics (article) | Khan Academy
An open system can exchange both energy and matter with its surroundings. · A closed system, on the other hand, can exchange only energy with its surroundings, …
A system where there is exchange of energy but not of mass is …
In thermodynamics, a closed system can exchange energy (as heat or work) but not matter, with its surroundings. An isolated system cannot exchange any heat, …
7.6: The First Law of Thermodynamics – Chemistry LibreTexts
An isolated system exchanges neither energy nor matter with the surroundings. A truly isolated system does not actually exist, however, because …
The Laws of Thermodynamics | Biology I – Simple Book …
The matter and its environment relevant to a particular case of energy transfer are classified as a system, and everything outside of that system is called …
What is entropy in thermodynamics?
entropy, the measure of a system’s thermal energy per unit temperature that is unavailable for doing useful work. Because work is obtained from ordered molecular motion, the amount of entropy is also a measure of the molecular disorder, or randomness, of a system.
What is an example of an isolated system?
A thermos flask is the best example of an isolated system. A thermos flask is used to keep things either cold or hot. Thus a thermos does not allow energy for transfer.
Which of the following exchanges with the surrounding takes place in a closed system?
In thermodynamics, a closed system can exchange energy (as heat or work) but not matter, with its surroundings. An isolated system cannot exchange any heat, work, or matter with the surroundings, while an open system can exchange energy and matter.
What type of system allows energy but not matter to enter and exit?
A closed system allows energy (usually heat) to be exchanged but not matter. An open system allows matter and energy to be freely exchanged.
What can be exchanged with the surroundings in a closed system?
A closed system can exchange energy with its surroundings through heat and work transfer. In other words, work and heat are the forms that energy can be transferred across the system boundary.
Open System, Closed System and Isolated System – Thermodynamics Physics
Images related to the topicOpen System, Closed System and Isolated System – Thermodynamics Physics
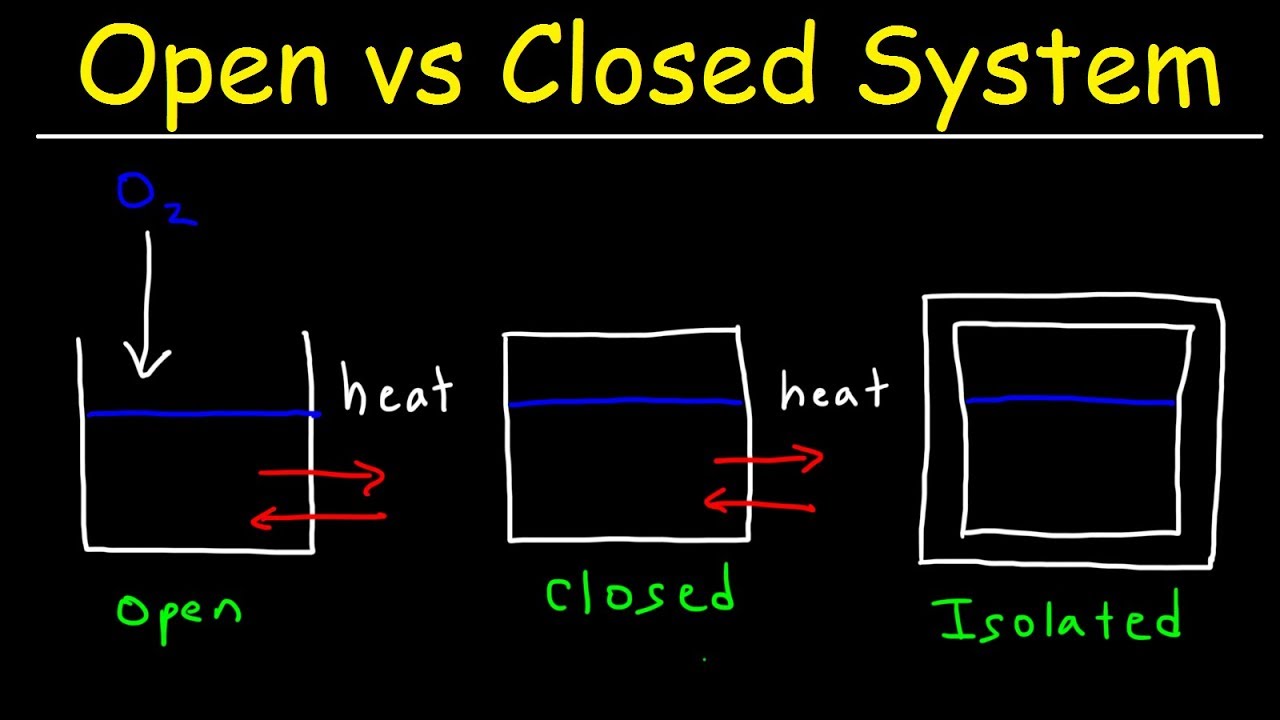
What is difference between isolated and adiabatic system?
Definition. Adiabatic System: An adiabatic system is a system that has no exchange of energy or matter with the surrounding environment. Isolated System: An isolated system is a system that has no transfer of energy and matter through its boundaries and has no surrounding environment.
What is entropy in biology?
Entropy is a measure of randomness or disorder in a system. Gases have higher entropy than liquids, and liquids have higher entropy than solids. An important concept in physical systems is that of order and disorder (also known as randomness).
What do you mean by thermodynamic process?
A thermodynamic process can be defined as a change or alteration from one type of equilibrium microstate to another type of system. The process can be interpreted by the initial and final states of the system.
What is conduction and convection and radiation?
Conduction is the transfer of thermal energy through direct contact. Convection is the transfer of thermal energy through the movement of a liquid or gas. Radiation is the transfer of thermal energy through thermal emission.
What is the difference between conduction and convection?
In conduction, heat transfer occurs between objects by direct contact. In convection, the heat transfer takes within the fluid. In radiation, heat transfer occurs through electromagnetic waves without involving particles. The heat transfer takes place due to the difference in temperature.
What is moving in convection?
Convection is the transfer of thermal energy by particles moving through a fluid. Thermal energy is always transferred from an area with a higher temperature to an area with a lower temperature. Moving particles transfer thermal energy through a fluid by forming convection currents.
What is the separate system and surrounding called?
Boundary: Anything which separates the system from its surrounding is called boundary.
What is a system and surroundings?
The system is the collective substances in the reaction such as the reactants and products. The surroundings are everything around the reaction such as the reaction flask and the room. During a reaction, energy is transferred between the system and surroundings.
What is reversible and irreversible process in thermodynamics?
In terms of thermodynamics, a reversible process is where the participants go back to its initial form by inculcating minor or negligible changes in their surroundings. Contrarily, an irreversible process is a naturally occurring phenomenon, which does not go back to its original state.
What is a closed and isolated system?
A closed system does not allow matter to enter or leave, but does allow energy to enter or leave. A covered pot on the stove is approximately a closed system. An isolated system does not allow either matter or energy to enter or leave. A thermos or cooler is approximately an isolated system.
Internal Energy and transfer of energy between system and surrounding
Images related to the topicInternal Energy and transfer of energy between system and surrounding

Which of the following is transferred in isolated system matter heat Both None of the both?
In thermodynamics, a closed system can exchange energy (as heat or work) but not matter, with its surroundings. An isolated system cannot exchange any heat, work, or matter with the surroundings, while an open system can exchange energy and matter.
In which thermodynamic process is there no flow of heat between the system and the surrounding?
Detailed Solution
The thermodynamic process in a system, during which no heat transfer occurs between thermodynamic systems and surrounding is called an adiabatic process. EXPLANATION: In an adiabatic process, no heat flows between the system and surroundings.
Related searches to When an exchange of energy happens with the surroundings and the system but matter is not transferred from or to the system this type of system is?
- open vs closed system physics
- 5 examples of open system in thermodynamics
- open system and closed system examples
- open system examples
- isolated system in thermodynamics
- closed system in thermodynamics
- example of open system in thermodynamics
- thermodynamic system
Information related to the topic When an exchange of energy happens with the surroundings and the system but matter is not transferred from or to the system this type of system is?
Here are the search results of the thread When an exchange of energy happens with the surroundings and the system but matter is not transferred from or to the system this type of system is? from Bing. You can read more if you want.
You have just come across an article on the topic When an exchange of energy happens with the surroundings and the system but matter is not transferred from or to the system this type of system is?. If you found this article useful, please share it. Thank you very much.