Are you looking for an answer to the topic “Which electron has highest electron affinity in periodic table?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Which Element Has the Highest Electron Affinity? Chlorine has the highest electron affinity among the elements. Its high affinity can be attributed to its large atomic radius, or size. Because chlorine’s outermost orbital is 3p, its electrons have a large amount of space to share with an incoming electron.Out of all the groups present in the periodic table, halogens have high electron affinity compared to others. This is due to the small size of all halogen atoms and high effective nuclear charge present on the nucleus.Thus, nonmetals have a higher electron affinity than metals, meaning they are more likely to gain electrons than atoms with a lower electron affinity. For example, nonmetals like the elements in the halogens series in Group 17 have a higher electron affinity than the metals. This trend is described as below.
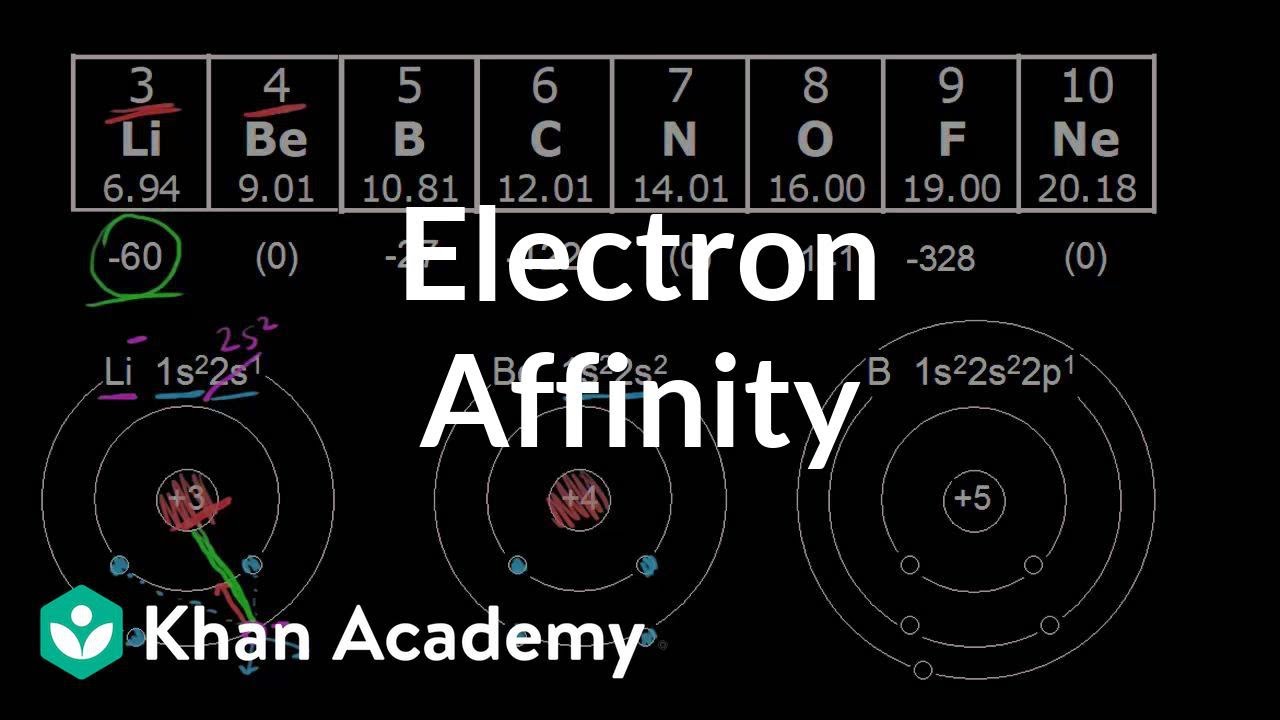
Which group in the periodic table has the highest electron affinity and why?
Out of all the groups present in the periodic table, halogens have high electron affinity compared to others. This is due to the small size of all halogen atoms and high effective nuclear charge present on the nucleus.
Which electrons have a higher electron affinity?
Thus, nonmetals have a higher electron affinity than metals, meaning they are more likely to gain electrons than atoms with a lower electron affinity. For example, nonmetals like the elements in the halogens series in Group 17 have a higher electron affinity than the metals. This trend is described as below.
Electron affinity: period trend | Atomic structure and properties | AP Chemistry | Khan Academy
Images related to the topicElectron affinity: period trend | Atomic structure and properties | AP Chemistry | Khan Academy

Which group has the highest affinity?
Halogens generally have the highest electron affinity.
Which has higher electron affinity F or Cl?
Electronegativity of fluorine is greater than that of chlorine but electron affinity of chlorine is greater than that of fluorine.
What is electron affinity in periodic table?
Key Points. The electron affinity of an atom or molecule is the propensity for that particle to gain an electron. This is an exothermic process for all non-noble gas elements. There are general trends in electron affinity across and down the periodic table of elements.
Where is the lowest electron affinity on the periodic table?
Chlorine has the highest electron affinity while mercury has the lowest. Electron affinity generally increases across a period (row) in the periodic table, due to the filling of the valence shell of the atom.
Which has higher electron affinity fluorine or neon?
Thus, noble gases have the least electron affinity in a period. Hence, we can conclude that in a period, fluorine (halogen) has higher electron affinity than neon (noble gas).
See some more details on the topic Which electron has highest electron affinity in periodic table? here:
Why Does Chlorine Have the Highest Electron Affinity?
Therefore, chlorine has a higher electron affinity than fluorine, and this orbital structure causes it to have the highest electron affinity of all of the …
Electron Affinity – Chemistry LibreTexts
Electron affinity increases upward for the groups and from left to right across periods of a periodic table because the …
Electron Affinity – The Periodic Table Variations Of Chemical …
Atoms, such as Group 7 elements, whose anions are more stable than neutral atoms have a higher electron affinity. The electron affinities of the noble gases …
Electron Affinity | Introduction to Chemistry
Atoms, such as Group 7 elements, whose anions are more stable than neutral atoms have a higher Eea. The electron affinities of the noble gases have not been …
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity
Images related to the topicThe Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity

Which element has the highest electron affinity in period 2?
Fluorine is the element which has high electron affinity.
Which alkali metal has the highest electron affinity?
Therefore, of the five alkali metals in the figure, the element that is expected to have the greatest electron affinity is lithium.
Which has more electron affinity F minus or Cl minus?
Electron affinity of Cl is greater than that of F.
Which has greater electron affinity fluorine or bromine?
…
Electron Affinity (decreases down the group)
| Halogen | Electron Affinity (kJ/mol) |
|---|---|
| Bromine | -324.6 |
| Iodine | -295.2 |
| Astatine | -270.1 |
Which has more electron affinity N or P?
Solution : Electron affinity, in general decreases down a group. However, electron affinity of P(70) is more than N(0). It is due to the releatively compact 2p-subshell. Thus, electron affinity of P is more than N and As.
Which element has the highest electron affinity most negative value )?
Electron affinity of non metals is highest negative. Here, apart from Br all are metals. So, Br has most negative electron affinity.
Electron Affinity Trend, Basic Introduction, Chemistry
Images related to the topicElectron Affinity Trend, Basic Introduction, Chemistry
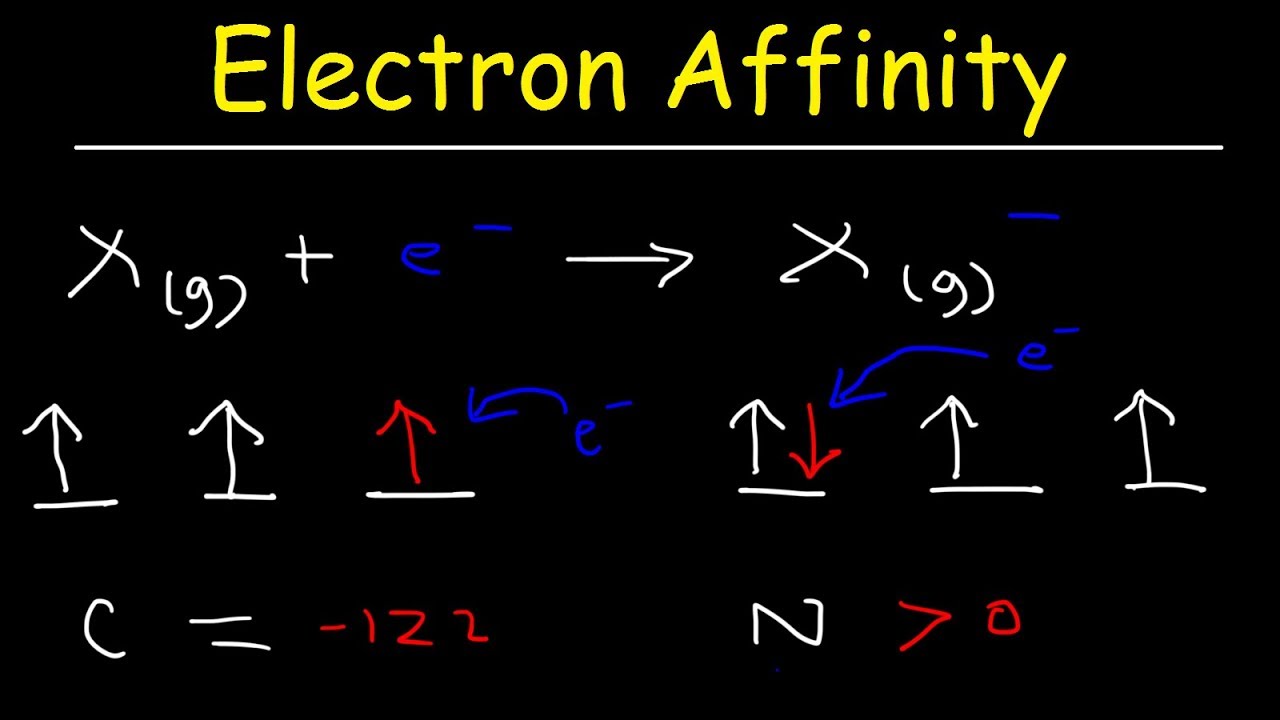
What does a high electron affinity mean?
Based on this sign convention, this means that a higher electron affinity indicates that an atom more easily accepts electrons. A lower electron affinity indicates that an atom does not accept electrons as easily.
Why do halogens have the highest electron affinity?
The halogens, which belong to Group VIIA, have the highest electron affinities because adding an electron to an atom results in a totally filled shell. Hence halogens have the greatest electron affinity.
Related searches to Which electron has highest electron affinity in periodic table?
- which element has highest electron affinity
- electron affinity trend down a group
- chlorine electron affinity
- electron affinity trend
- why chlorine has highest electron affinity
- which group has the highest electron affinity within a period
- electron affinity table
- which element has the lowest electron affinity
Information related to the topic Which electron has highest electron affinity in periodic table?
Here are the search results of the thread Which electron has highest electron affinity in periodic table? from Bing. You can read more if you want.
You have just come across an article on the topic Which electron has highest electron affinity in periodic table?. If you found this article useful, please share it. Thank you very much.