Are you looking for an answer to the topic “Which element has the electron configuration of 2/8 3?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
The element with the Electron Configuration (2,8,3) is Aluminum as it has the atomic number 13.Solution : Atomic number of the element (Z) = 2 + 8 + 3 = 13 <br> (i) The element belongs to group =13 (ii) The element belongs to period = 3.Potassium with atomic number 19 has the electronic configuration (2,8,8,1). So, it can lose one extra electron and can become a positive ion. So, it is a metal.
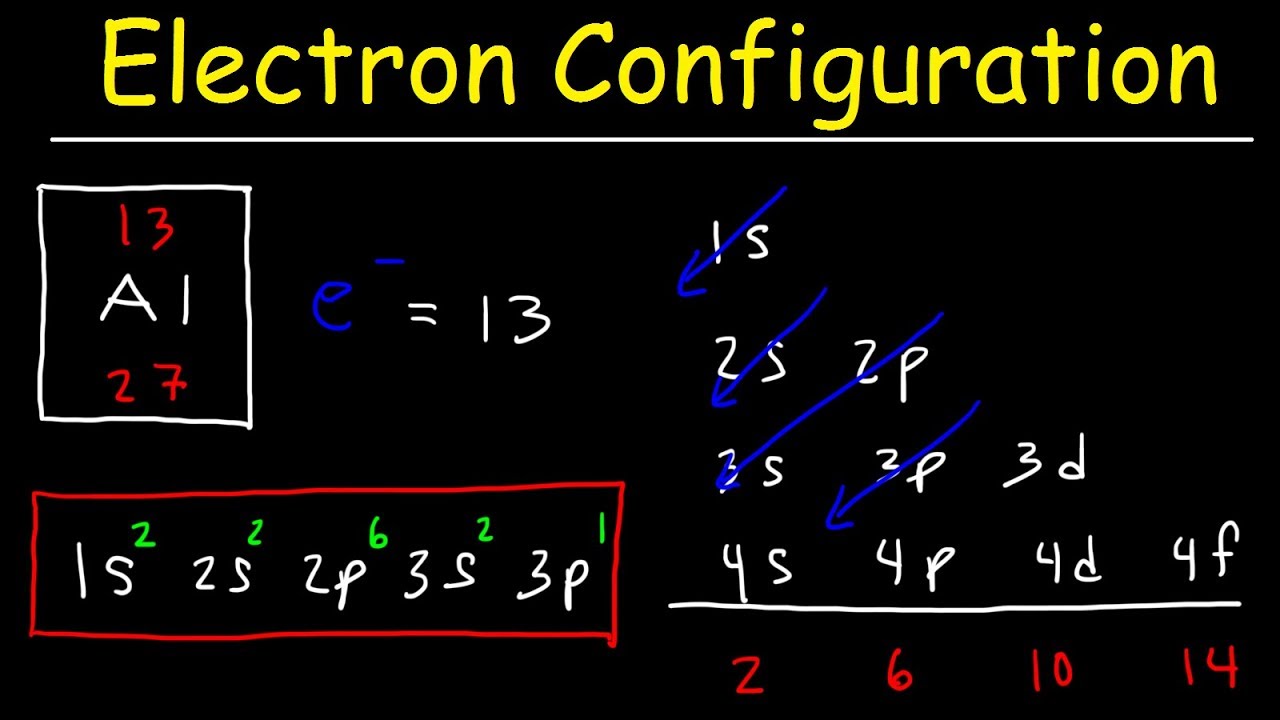
What is the period number of electronic configuration 2 8 3?
Solution : Atomic number of the element (Z) = 2 + 8 + 3 = 13 <br> (i) The element belongs to group =13 (ii) The element belongs to period = 3.
What is the name of an element whose electronic configuration is 2/8 8?
Potassium with atomic number 19 has the electronic configuration (2,8,8,1). So, it can lose one extra electron and can become a positive ion. So, it is a metal.
Electron Configuration – Basic introduction
Images related to the topicElectron Configuration – Basic introduction

Which element has 2 and 8 and 8 and 2 electron configuration?
The electronic configuration of calcium is 2,8,8,2.
What is the valency of elements with electronic configuration 2 8 3?
Aluminium is an element with valency is 3 having the electronic configruration is 2, 8, 3.
Is the electronic configuration of an atom is 2 8 3 Then the formula of its chloride will be?
Formula of its chloride is AlCl3.
Which element has the electron configuration 2 8 2?
Magnesium has the electronic configuration 2,8,2.
Which of the following is the valency of an element if it has 2 8 2 electronic configuration?
Answer. b. The valency of it is +2.
See some more details on the topic Which element has the electron configuration of 2/8 3? here:
Which element has :The electronic configuration 2 , 8 , 3 ?
Aluminum · An element has electronic configuration 2,8,7. What is atomic number of this element? · The element having the electronic configuration 1s22s22p63s23p1 …
an element has electronic configuration 2, 8, 3 what is the …
Atomic number of the element = 2+8+3=13, so it is aluminum. Aluminum belongs to the p block element , having electronic configuration …
an element has electronic configuration 2 8 3 what is the …
An element has electronic configuration 2, 8, 3. What is the atomic number of this element? To which (i) group and (ii) period this element …
Electron configurations of the elements (data page) – Wikipedia
1s2s2p1s22s12111s22s2View 351 more rows
What type of element an element with an electronic structure of 2 8 1 is likely to be?
…
Electronic structures and the periodic table.
| Electronic structure feature | Link to the periodic table |
|---|---|
| Number of shells | Period number |
What is the electron configuration of Zn?
How will you identify the position of an element with electronic configuration 2 8 2 identify the element?
Element with electronic configuration 2,8,2 belongs to 3rd period and 2nd group. The valence electrons give us the idea of group numbers. As the electrons are divided into 3 different shell therefore the period number is 3.
What is the electron configuration of Aluminium?
Where would you locate the element with electronic configuration 2 8 in the modern periodic table?
The element with electronic configuration 2, 8 has a complete octet. Therefore, it is a noble gas. It will be found in group 18 with all the other noble gases.
Why electronic configuration of potassium calcium are 2,8,8,1 and 2,8,8,2 why not 2,8,9 2,8,10
Images related to the topicWhy electronic configuration of potassium calcium are 2,8,8,1 and 2,8,8,2 why not 2,8,9 2,8,10

Which element has the electron configuration of 2/8 3?
The element with the Electron Configuration (2,8,3) is Aluminum as it has the atomic number 13.
What is the electronic configuration of 13al?
The electron configuration for Aluminum is 1s22s22p63s23p1.
What is electronic configuration of scandium?
What will be the formula of the chloride of the element with electronic configuration 2 8 4?
The element with electronic configuration is 2, 8, 4 is Si and with chloride it forms SiCl4.
What element has the electron arrangement of 2/8 5?
1 Expert Answer
If that is the complete electron configuration, then we are looking at a total of 15 electrons, making this element phosphorous (P).
What is the configuration of sodium?
What is the atomic number of this element 2 8 4?
The given electronic configuration 2, 8, 4. Hence there are two electrons in the K shell, eight electrons in the L shell and four electrons in the M shell. Thus the total number of electrons is 14. – Since the atomic number is fourteen we can assume that the given element is silicon.
What is the atomic number of this element 2 8 1?
Answer: Potassium with atomic number 19 has the electronic configuration (2,8,8,1).
What is the atomic number of this element 2 8 7?
⇒ 2+8+7 = 17. Therefore, the atomic number of this element is 17 and the element is chlorine.
What is the valency of an element having electronic configuration 2 8 8?
Answer. Answer: The valency of it is +2. … Be because they both have same number of valence electron in their outermost orbit.
GCSE Chemistry – Electron Arrangement #8
Images related to the topicGCSE Chemistry – Electron Arrangement #8

What is the valency of cobalt?
| Element | Atomic Number | Valency |
|---|---|---|
| Valency of Cobalt | 27 | 3, 2 |
| Valency of Nickel | 28 | 2 |
| Valency of Copper (Cu) | 29 | 2, 1 |
| Valency of Zinc | 30 | 2 |
What is the valency of CaO?
The valency of Ca in the compound CaO is 2.
Related searches to Which element has the electron configuration of 2/8 3?
- 2,8,6 electron configuration
- 2 8 7 electron configuration
- full electron configuration
- 2, 8, 7 electron configuration
- which element has the following electronic configuration
- which element has the electron configuration
- 2 4 electron configuration
- 2 8+7 element
- 286 electron configuration
- which element has the electron configuration of 28 3 n
- 2 87 element
- 2 6 electron configuration
- identify the element based on its electron configuration 289
- electronic configuration of scandium
- which element has the electron configuration of 1s22s22p63s2
- which element has the electron configuration of 28 3 1
- which element has an electron configuration of 2.8.8
- which element has the electron configuration of 28 3 2
- which element has the electron configuration of 28 3 3
Information related to the topic Which element has the electron configuration of 2/8 3?
Here are the search results of the thread Which element has the electron configuration of 2/8 3? from Bing. You can read more if you want.
You have just come across an article on the topic Which element has the electron configuration of 2/8 3?. If you found this article useful, please share it. Thank you very much.