Are you looking for an answer to the topic “Which element has the most negative electron affinity?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Electron affinity of non metals is highest negative. Here, apart from Br all are metals. So, Br has most negative electron affinity.Therefore carbon has the highest (most negative) electron affinity rather than nitrogen. Which of the following has the largest atomic radius? Atomic radius increases going down a group (column) and to the left across a period (row) on the periodic table.Hence chlorine has the highest value of negative electron gain enthalpy among all of these.

Which element has the most negative electron affinity quizlet?
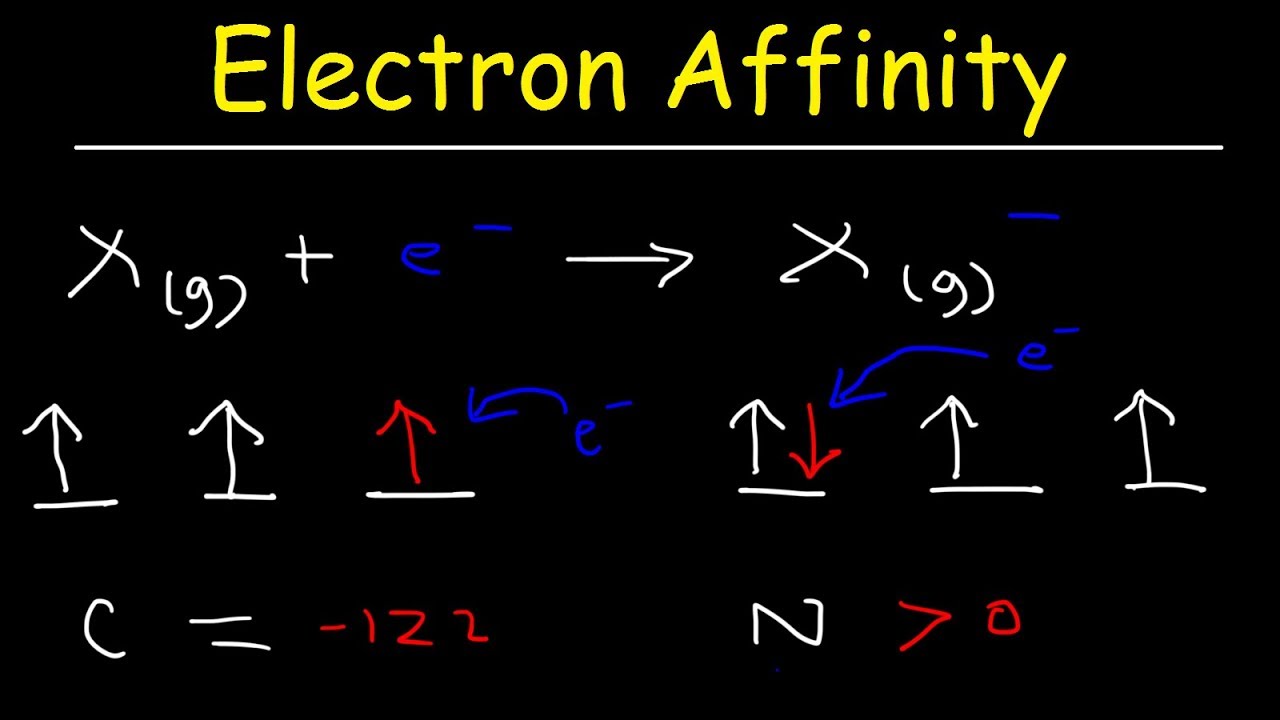
Therefore carbon has the highest (most negative) electron affinity rather than nitrogen. Which of the following has the largest atomic radius? Atomic radius increases going down a group (column) and to the left across a period (row) on the periodic table.
Which of the following element has highest negative?
Hence chlorine has the highest value of negative electron gain enthalpy among all of these.
Which element has the highest most negative electron affinity? A Xe B S C Cs D Ba E Cu
Images related to the topicWhich element has the highest most negative electron affinity? A Xe B S C Cs D Ba E Cu

Which element has the highest most negative electron affinity group of answer choices Li KR CR mg S?
Given the remaining choices, the element with the highest electron affinity is thus sulfur (S).
What does a higher negative number mean with electron affinity?
A more negative electron affinity corresponds to a greater attraction for an electron. (An unbound electron has an energy of zero.) Trends: As with ionization energy, there are two rules that govern the periodic trends of electron affinities: Electron affinity becomes less negative down a group.
Which has more electron affinity carbon or nitrogen?
Nitrogen has lower electron affinity than its preceeding element carbon because. Electron affinity decreases along a period.
How do you find electron affinity?
The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. X ( g ) + e − → X − ( g ) + E . A . Therefore, the electron affinity of chlorine is – 349 KJ/mol.
Which has highest negative electron gain enthalpy?
Halogens have the highest negative value of electron gain enthalpy. Energy is released when an electron is added to the atom.
See some more details on the topic Which element has the most negative electron affinity? here:
7.5: Electron Affinities – Chemistry LibreTexts
Fluorine, therefore, has a lower affinity for an added electron than does chlorine. Consequently, the elements of the third row (n = 3) have the …
Periodic Trends — Electron Affinity
The halogens in Group 7A all have large negative electron affinities, since they are only one electron away from having a noble gas configuration, …
What Is Electron Affinity? | Trends & Chart | ChemTalk
Chlorine has the highest electron affinity among the elements. Its high affinity can be attributed to its large atomic radius, or size.
What element has the most negative electron affinity?
Which element has the most negative first electron affinity? · It is represented by this chemical equation: · Comparing Cl, Br, and I which are …
Which one of the following element has the highest negative value for its electron gain enthalpy se o/s te?
O will have the most negative value of electron gain enthalpy due to its small size and electronic gain repulsion and under given is the correct order of negative value of electron gain enthalpy.
Which of the following elements has the most negative gain enthalpy?
The element (chlorine) corresponding to the electronic configuration (iii) will have more negative electron gain enthalpy.
What has the lowest electron affinity?
The correct answer is Argon. Argon has all filled orbitals as well as a filled valence shell. As a result, it doesn’t want to lose or gain any electrons. Hence, argon has the lowest electron affinity.
Why do halogens have the most negative electron affinities?
All the halogens have values of about 300 kJ mol–1 while the group VI nonmetals have somewhat lower values, in the region of 200 kJ mol–1 or less. The high electron affinities of the halogens are a result of their having an almost complete outer shell of electrons.
Electron Affinity Trend, Basic Introduction, Chemistry
Images related to the topicElectron Affinity Trend, Basic Introduction, Chemistry
How do you find the lowest electron affinity?
The less valence electrons an atom has, the least likely it will gain electrons. Electron affinity decreases down the groups and from right to left across the periods on the periodic table because the electrons are placed in a higher energy level far from the nucleus, thus a decrease from its pull.
Does chlorine or bromine have a more negative electron affinity?
Chlorine does have a higher electron affinity than bromine. So, why does that happen? As you know, both chlorine and bromine are located in group 17 of the periodic table, which implies that they have seven electrons on their outermost shell.
Which electron has highest affinity?
Halogens has higher electron affinity and it is supposed to be for fluorine, but chlorine has higher electron affinity than fluorine due to fluorine’s smaller size. Hence, among given options chlorine has highest electron affinity.
Which has the more negative electron affinity the bromine atom or the Br ion?
Which has the more negative electron affinity Br or Br −? Stability means lowering energy, so to become stable, which bromine wants, is to release energy. Thus, a neutral bromine atom has a very negative ΔE. By contrast, the bromide ion, Br-, already has this extra electron.
Why does nitrogen have a negative electron affinity?
This means that the incoming electron will experience significant repulsion compared with when it’s added to an empty orbital. As a result, you need energy to add an electron to nitrogen, and hence its electron affinity is actually negative.
Are electron affinities always negative?
While first electron affinities can be negative, positive, or zero, second electron affinities are always positive.
Which has more electron affinity carbon or oxygen?
The electron affinity decreases from left to right in a period. Carbon comes first when we go from left to right. So electron affinity of carbon is greater than oxygen.
How do you know if electron affinity is positive or negative?
Remember that the definition of an electron affinity is the energy released, so that means that the reaction is exothermic. If a reaction is exothermic, the change in energy is negative. This means that the electron affinity is positive.
What is electron affinity in periodic table?
Key Points. The electron affinity of an atom or molecule is the propensity for that particle to gain an electron. This is an exothermic process for all non-noble gas elements. There are general trends in electron affinity across and down the periodic table of elements.
Why electron affinity of chlorine is more than fluorine?
the electron affinity of the fluorine is less than chlorine because the size of fluorine is too small as size decreases from left to right inside period, whereas chlorine has a larger size to accommodate electrons hence electron affinity of chlorine is more than fluorine.
Electron Affinity – Chemistry Tutorial
Images related to the topicElectron Affinity – Chemistry Tutorial

Which element has the most negative electron gain enthalpy and why?
Hence, the element with the most negative electron gain enthalpy is chlorine, and the one with the least negative electron gain enthalpy is phosphorus.
Which of the following will have the most negative electron affinity and which one has the least?
Cl has the most negative one. P has the least negative one due to the larger size.
Related searches to Which element has the most negative electron affinity?
- which element has the most favorable (most negative) electron affinity
- electron affinity chart
- electron affinity of al
- which elements have negative electron affinity
- which of the following elements has the most negative electron affinity
- electron affinity of as
- which element has the highest electron affinity
- which element has the most negative electron affinity
- which element has the most negative electron affinity quizlet
- how to determine which element has the most negative electron affinity
- which element has the most negative electron affinity li be one h
- which element has the highest (most negative) electron affinity
- which has the most negative electron affinity
- which element has the most negative electron affinity be li ne o
- electron affinity of k
- electron affinity of na
- electron affinity of chlorine
- which element has the lowest electron affinity
Information related to the topic Which element has the most negative electron affinity?
Here are the search results of the thread Which element has the most negative electron affinity? from Bing. You can read more if you want.
You have just come across an article on the topic Which element has the most negative electron affinity?. If you found this article useful, please share it. Thank you very much.