Are you looking for an answer to the topic “Which elements have no affinity for electrons?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Noble gases or inert gases do not have a tendency to gain an electron. Hence, its electron affinity is zero.Noble gases have zero electron affinity, this is because they have a stable fulfilled electronic configuration.Electron affinities are the negative ion equivalent, and their use is almost always confined to elements in groups 16 and 17 of the Periodic Table.
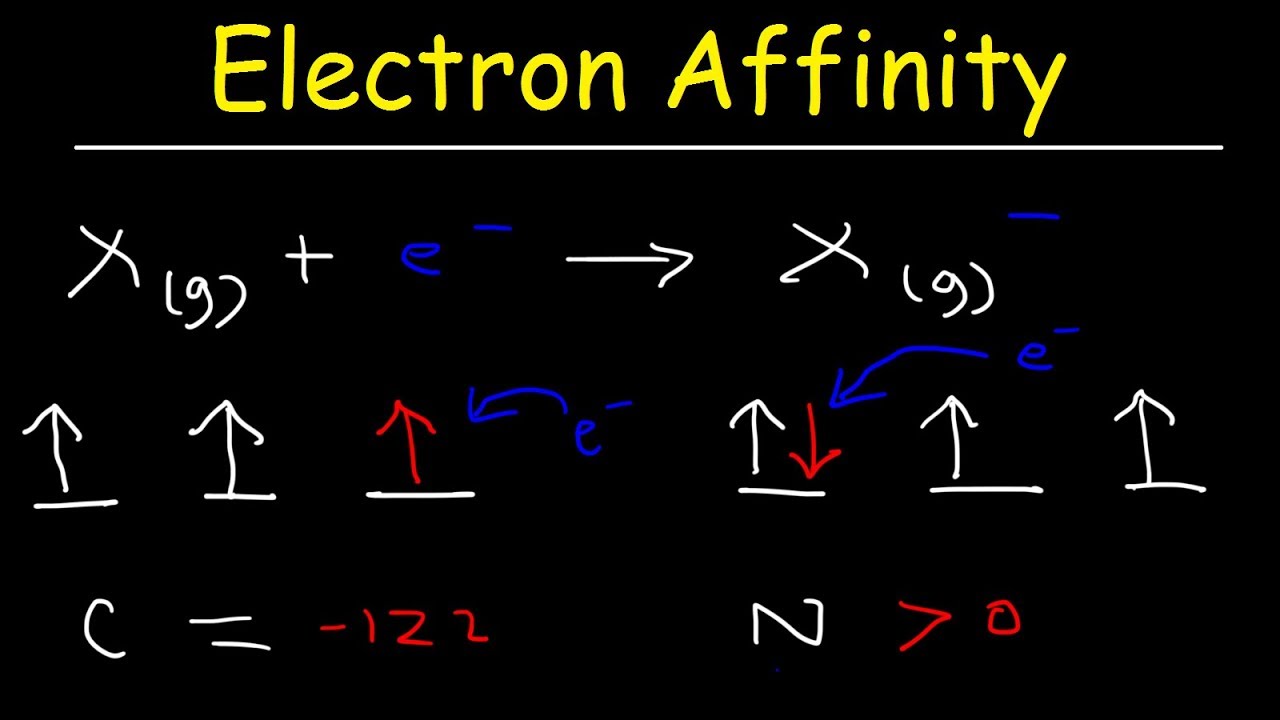
Which type of elements have no affinity for electrons?
Noble gases have zero electron affinity, this is because they have a stable fulfilled electronic configuration.
Do all elements have electron affinity?
Electron affinities are the negative ion equivalent, and their use is almost always confined to elements in groups 16 and 17 of the Periodic Table.
Electron Affinity Trend, Basic Introduction, Chemistry
Images related to the topicElectron Affinity Trend, Basic Introduction, Chemistry

Why do some elements have 0 electron affinity?
Since the Noble Gases already have that ‘perfect status’ then they have an affinity of 0. Affinity is the change in energy of the atom when an electron is added. Noble Gases are at the perfect number of 8 electrons. They don’t ‘want’ anymore electrons, so there’s zero change in the energy of the atom.
What does 0 electron affinity mean?
Unlike ionization energies, which are always positive for a neutral atom because energy is required to remove an electron, electron affinities can be negative (energy is released when an electron is added), positive (energy must be added to the system to produce an anion), or zero (the process is energetically neutral) …
Why does nitrogen have no electron affinity?
Nitrogen has a zero value of electron affinity because of the stability of the half filled 2psubshell (that is, N has little tendency to accept another electron)
Do noble gases have electron affinity?
Any electrons added to a noble gas would have to be the first electron in a new (larger) energy level. This causes the noble gases to have essentially zero electron affinity.
What has the lowest electron affinity?
The correct answer is Argon. Argon has all filled orbitals as well as a filled valence shell. As a result, it doesn’t want to lose or gain any electrons. Hence, argon has the lowest electron affinity.
See some more details on the topic Which elements have no affinity for electrons? here:
Periodic Trends — Electron Affinity
The electron affinity of an element is the energy change which accompanies the addition of an electron to an atom in the gas phase to produce a negatively …
What Is Electron Affinity? | Trends & Chart | ChemTalk
Chlorine has the highest electron affinity among the elements. Its high affinity can be attributed to its large atomic radius, or size. Because …
Electron Affinity of The Elements – Breaking Atom
Of the metals, mercury has the lowest electron affinity. 3. Is electron affinity the same as electronegativity? No. Electron affinity is related to …
What is the electron affinity of Lithium?
The electron affinity of lithium is 59.6 kJ mol‑1. The ionisation energies of lithium are given below.
Which element has least electronegativity?
The least electronegative elements are cesium (Cs) and francium (Fr), with electronegativity values of 0.7. Therefore, Fluorine is the most electronegative element and cesium is the least electronegative element.
Why do noble gases have zero electron affinity?
Group VIII elements, noble gases, have electron affinities near zero, since each atom possesses a stable octet and will not accept an electron readily.
Which of the following pair of elements will have electron affinity 0?
Noble gases or inert gases do not have a tendency to gain an electron. Hence, its electron affinity is zero.
Does neon have electron affinity?
The electron affinity of neon is 0 kJ mol‑1.
Electron affinity: period trend | Atomic structure and properties | AP Chemistry | Khan Academy
Images related to the topicElectron affinity: period trend | Atomic structure and properties | AP Chemistry | Khan Academy
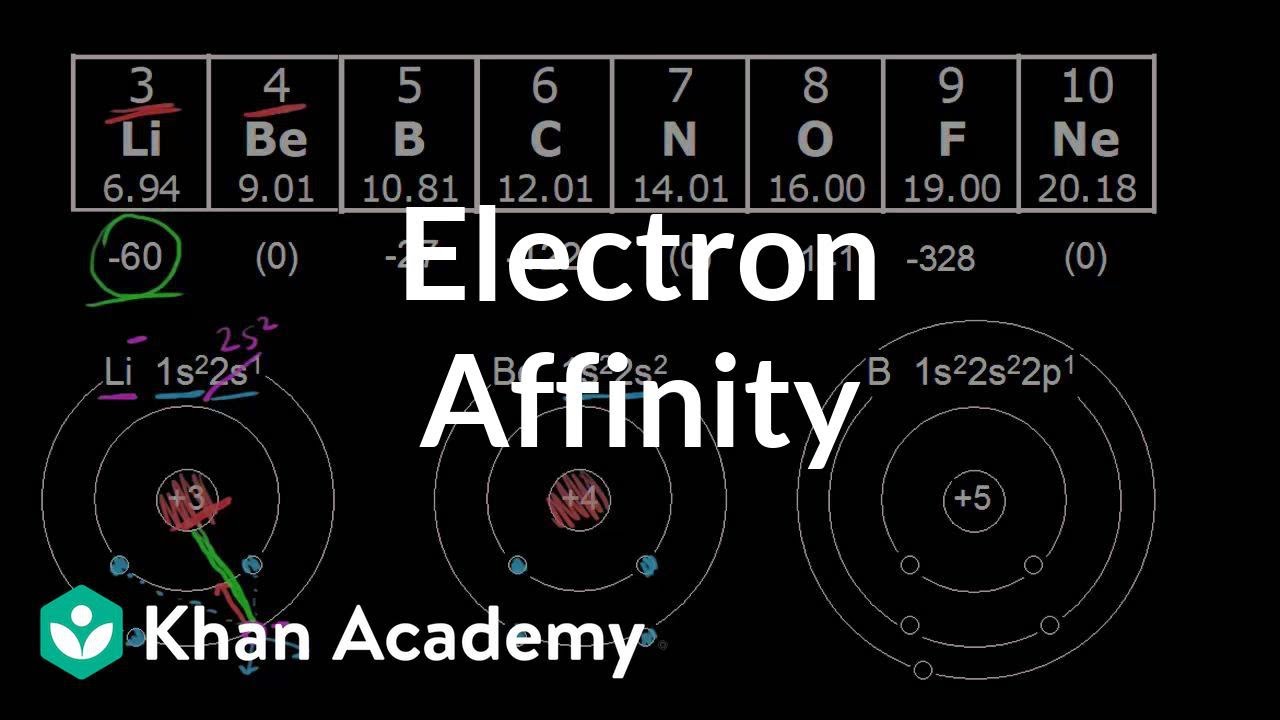
What is the electron affinity of beryllium?
…
Elements.
| Z | 4 |
|---|---|
| Element | Be |
| Name | Beryllium |
| Electron affinity (eV) | -0.5(2) |
| Electron affinity (kJ/mol) | -48(20) |
What is the electron affinity of bromine?
Electron Affinity of Bromine is 324.6 kJ/mol.
What is the electron affinity of carbon?
The electron affinity of carbon is 153.9 kJ mol‑1.
What is the electron affinity of boron?
Electron Affinity of Boron is 26.7 kJ/mol.
What is the electron affinity of fluorine?
Electron Affinity of Fluorine is 328 kJ/mol.
Why electron affinity of beryllium magnesium and nitrogen are almost zero?
Be 1s22s2 has a filled valence shell, so it does not have the tendency to gain electrons. Thus its electron affinity is almost zero.
Why do group 18 elements have zero electron affinity?
The elements of group 18 (noble gases) have zero electron affinity due to their stable electronic configuration.
Do halogens have high electron affinity?
Halogens, like bromine, chlorine and fluorine, have a high electron affinity (a high attraction toward obtaining another electron), as they have 7… See full answer below.
Does fluorine have the highest electron affinity?
Fluorine, which is higher up the group then chlorine, has a lower electron affinity. This is because the electrons in the outermost shell of a fluorine atom are closer together.
Which element has the most electron affinity?
Chlorine has the highest electron affinity among the elements. Its high affinity can be attributed to its large atomic radius, or size. Because chlorine’s outermost orbital is 3p, its electrons have a large amount of space to share with an incoming electron.
What is electron affinity? | Chemistry | Extraclass.com
Images related to the topicWhat is electron affinity? | Chemistry | Extraclass.com

Why does Mercury have the lowest electron affinity?
Metals: Metals like to lose valence electrons to form cations to have a fully stable shell. The electron affinity of metals is lower than that of nonmetals. Mercury most weakly attracts an extra electron.
Which of the following elements has the maximum electron affinity?
Although Fluorine has the highest electronegativity, Chlorine has the highest electron affinity and this is because of the considerable repulsion in the tightly packed 2p subshell of Fluorine.
Related searches to Which elements have no affinity for electrons?
- what element has the lowest electron affinity
- which elements has the lowest electron affinity
- which element has highest electron affinity
- what elements have the lowest electron affinity
- electron affinity of beryllium and boron
- which of the following elements has the most negative electron affinity
- elements with zero electron affinity
- electron affinity trend
- which elements have no affinity for electrons
- argon electron affinity
- electron affinity of o
Information related to the topic Which elements have no affinity for electrons?
Here are the search results of the thread Which elements have no affinity for electrons? from Bing. You can read more if you want.
You have just come across an article on the topic Which elements have no affinity for electrons?. If you found this article useful, please share it. Thank you very much.