Are you looking for an answer to the topic “Which Is The Electron Configuration For Lithium 1s2?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Keep Reading

Which element has the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d6?
Thus, following the rules on how to fill the orbitals, the electronic configuration of iron (for example) is 1s2 2s2 2p6 3s2 3p6 4s2 3d6 , and it is abbreviated form [Ar] 4s2 3d6.
How do I form Li +?
Now, the lithium cation, Li+ , is formed when lithium loses the electron located on its outermost shell → its valence electron. This electron is located on the second energy level, in the 2s-orbital.
Lithium Electron Configuration
Images related to the topicLithium Electron Configuration

What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d10?
| A | B |
|---|---|
| calcium | 1s2 2s2 2p6 3s2 3p6 4s2 |
| chromium | 1s2 2s2 2p6 3s2 3p6 4s1 3d5 ! |
| copper | 1s2 2s2 2p6 3s2 3p6 4s1 3d 10 ! |
| bromine | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 |
What element is 1s2 2s1?
| A | B |
|---|---|
| Lithium | 1s2 2s1 |
| Boron | 1s2 2s2 2p1 |
| Beryllium | 1s2 2s2 |
| Oxygen | 1s2 2s2 2p4 |
Has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d3?
Correct option a fifthExplanation :The electronic configuration of an atom 1s2 2s1 2p6 3s2 3p6 3d3 4s2. In the configuration the last electron of the atom is filled in d sub-shell as 3d3. Thus this element belongs to d-block of the periodic table with group no. V.
What element has this electron configuration Xe 4f14 5d10 6s2 6p3 *?
| Element Atomic Number | Element Symbol | Element Electron Configuration |
|---|---|---|
| 80 | Hg | [Xe] 4f14 5d10 6s2 |
| 81 | Tl | [Xe] 4f14 5d10 6s2 6p1 |
| 82 | Pb | [Xe] 4f14 5d10 6s2 6p2 |
| 83 | Bi | [Xe] 4f14 5d10 6s2 6p3 |
What element has the electron configuration 1s2 2s2 2p3?
1. Element with electron configuration 1s2 2s2 2p3 is Nitrogen (N.) It has two electrons in 1s, two in 2s, and three in 2p (arbitrarily two in 2px and 1 in 2py.)
See some more details on the topic Which Is The Electron Configuration For Lithium 1s2? here:
Electron Configuration for Lithium (Li) – TerpConnect
Lithium is the third element with a total of 3 electrons. In writing the electron configuration for lithium the first two electrons will go in the 1s …
Why is the ground state electron configuration for Lithium 1s^22s
Abstract: The electronic ground state for Lithium is 1s^22s, … higher in energy then the configuration that includes the 2s electron.
Atomic Numbers and Electron Configurations 4 Flashcards
A. orbital size B. orbital mass C. orbital shape D. orbital orientation, Which is the electron configuration for lithium? A. 1s2 B. 2s3 C. 1s22s1 D. 1s12s2 …
Li+ Electron Configuration (Lithium Ion)
Images related to the topicLi+ Electron Configuration (Lithium Ion)

What is 1s 2s 2p 3s 3p?
1s 2s 2p 3s 3p represents the electron orbital energy levels.
What element has this electron configuration?
…
| Element | Atomic number | Electron configuration |
|---|---|---|
| argon | 18 | 1s22s22p63s23p6 |
Which of the following shows the electron configuration of Li?
The electron configuration of lithium is 1s22s1. The sum of the superscripts in an electron configuration is equal to the number of electrons in that atom, which is in turn equal to its atomic number.
How many electrons are in Li?
How many protons and electrons are in Li+?
How many protons and electrons are in Li+? Lithium atom has electron arrangement 2,1 and the Li+ ion will be 2. So 7/3 Li+ has 3 protons, 4 neutrons and 2 electrons.
Electron Configuration – Basic introduction
Images related to the topicElectron Configuration – Basic introduction
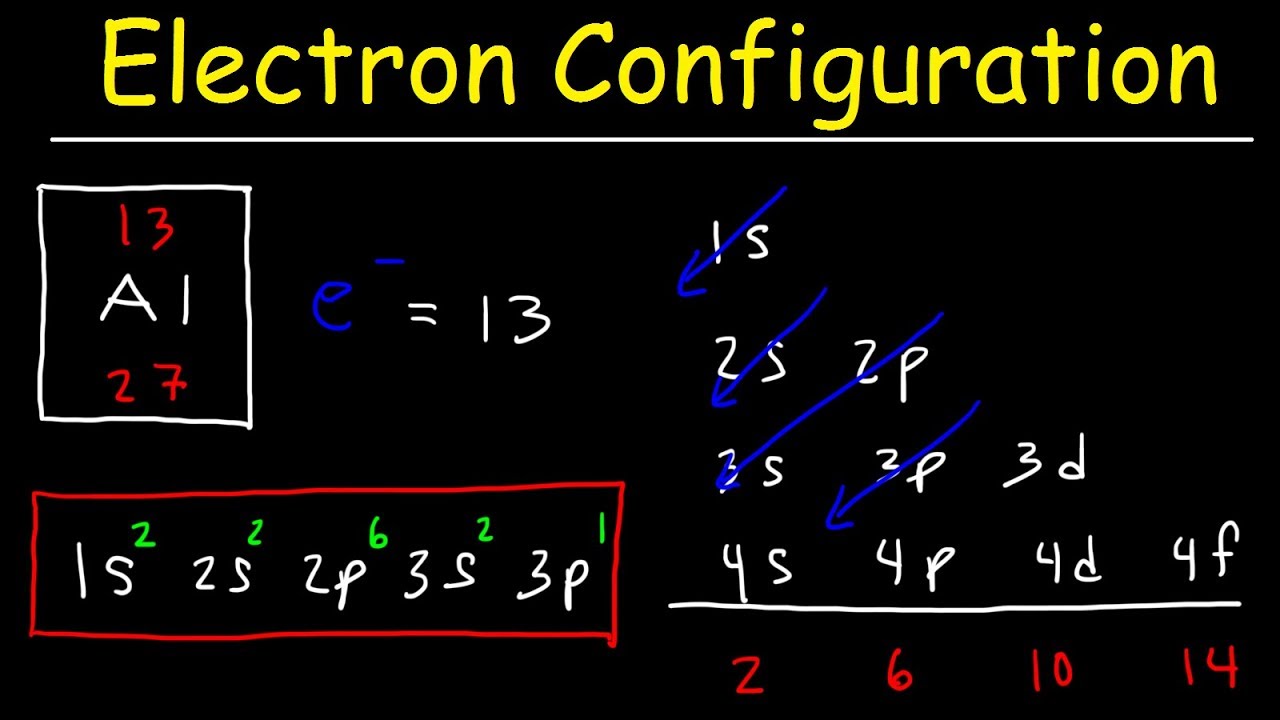
What is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6?
Kr (Krypton) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6. Rb (Rubidium) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1. Sr (Strontium)
What atom matches this electron configuration 1s2 2s2 2p6 3s3 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d9?
So, an antimony atom with charge +2 has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p1.
Related searches to Which Is The Electron Configuration For Lithium 1s2?
- which is the electron configuration for boron brainly
- write the electron configuration for f
- which is the electron configuration for boron 1s22s3 1s22s23s1 1s12s22p2 1s22s22p1
- what is the last electron configuration of magnesium
- rb electron configuration
- which is the electron configuration for boron? 1s22s3 1s22s23s1 1s12s22p2 1s22s22p1
- electron configuration of k
- which is the electron configuration for lithium 1s22s31s22s11s12s2
- electron configuration of magnesium
- Rb electron configuration
- which is the electron configuration for lithium 1s2
- how do you write the electron configuration for lithium
- what’s the electron configuration for lithium
- Electron configuration of Oxygen
- Electron configuration of magnesium
- which is the electron configuration for boron quizlet
- Write the electron configuration for f
Information related to the topic Which Is The Electron Configuration For Lithium 1s2?
Here are the search results of the thread Which Is The Electron Configuration For Lithium 1s2? from Bing. You can read more if you want.
You have just come across an article on the topic Which Is The Electron Configuration For Lithium 1s2?. If you found this article useful, please share it. Thank you very much.