Are you looking for an answer to the topic “Which is the mass of 8 moles of NaCl?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
1 Answer. The mass of eight moles of sodium chloride is ≈500g .One mol of NaCl (6.02 x1023 formulas) has a mass of 58.44 g.Answer: 1 mole is equal to 1 moles NaCl, or 58.44277 grams.
How many moles are in NaCl?
One mol of NaCl (6.02 x1023 formulas) has a mass of 58.44 g.
What is the mass of 10 moles of NaCl?
Answer: 1 mole is equal to 1 moles NaCl, or 58.44277 grams.
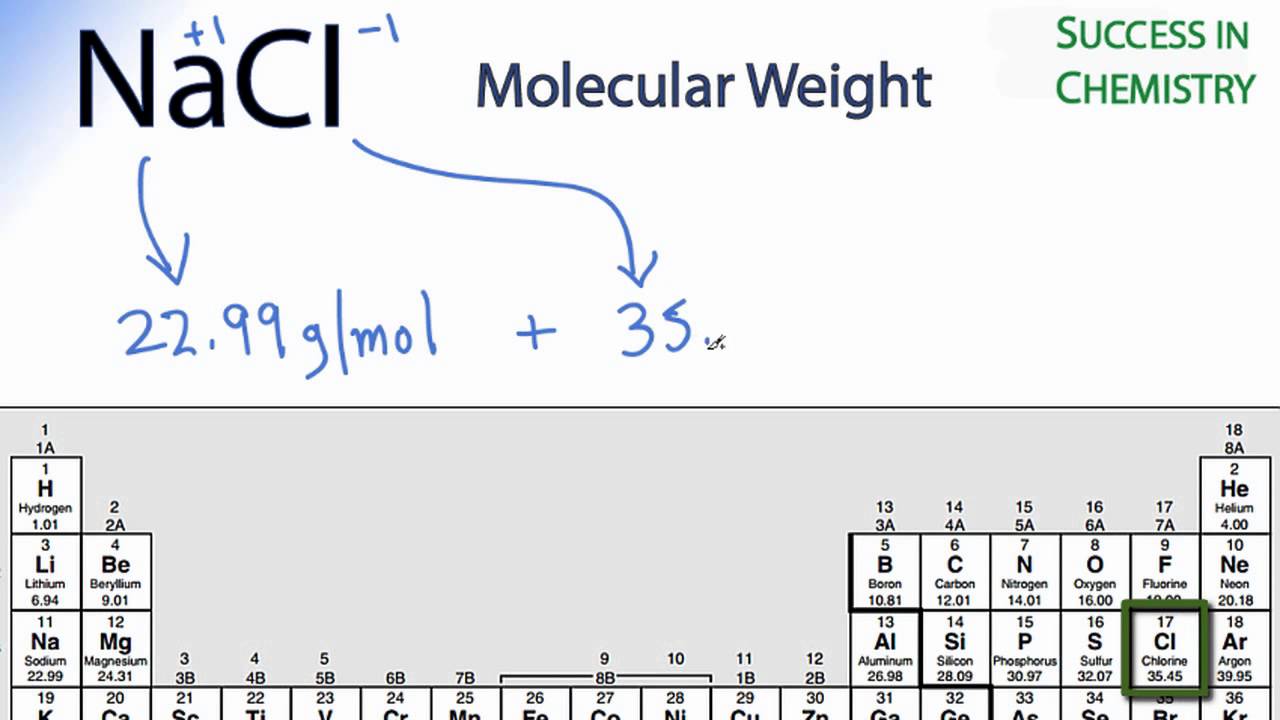
NaCl Molar Mass / Molecular Weight
Images related to the topicNaCl Molar Mass / Molecular Weight

Which is the mass of 5 moles of sodium chloride?
Answer: the mass of 5 mol of sodium chloride is exactly 71,75 grams.
How do I calculate moles?
- The formula for the number of moles formula is expressed as.
- Given.
- Number of moles formula is.
- Number of moles = Mass of substance / Mass of one mole.
- Number of moles = 95 / 86.94.
What is the mass of 4 moles of sodium chloride?
The molar mass of sodium chloride is 58.4 g/mol. This means that one mole of NaCl will weigh 58.4. 4 moles of NaCl will weigh 4 times as much.
How many moles are in 10g of NaCl?
Hence, 10 grams of NaCl is equivalent to 1/58.5×10 moles, which is equal to 0.17 moles of NaCl.
What is the mass of 10 mole of water?
So, 10 mole of water will weigh (18×10) = 180g.
See some more details on the topic Which is the mass of 8 moles of NaCl? here:
Convert moles NaCl to grams – Conversion of Measurement …
1 moles NaCl to grams = 58.44277 grams · 2 moles NaCl to grams = 116.88554 grams · 3 moles NaCl to grams = 175.32831 grams · 4 moles NaCl to grams = 233.77108 …
Which is the mass of 8 moles of sodium chloride? – Answers
Sodium chloride has a molar mass of about 58.5 g/mol. So multiply 8 moles by molar mass to get about 468 grams.
chemistry ch.10 Flashcards | Quizlet
which element has a molar mass of 30.974 g/mol … which is the mass of 8 moles of sodium chloride … how many formula units are in 3.6 grams of NaCl.
How do you find the mass?
One way to calculate mass: Mass = volume × density. Weight is the measure of the gravitational force acting on a mass. The SI unit of mass is “kilogram”. The SI unit of weight is Newton (N).
Concept of Mole – Part 1 | Atoms and Molecules | Don’t Memorise
Images related to the topicConcept of Mole – Part 1 | Atoms and Molecules | Don’t Memorise

How do you convert moles to grams?
- Calculate how many moles are mentioned in the question.
- Find the molar mass of the substance.
- Multiply both the values.
What is the mol of NaOH?
One mole of NaOH has a molar mass of 40.0 g, so 40.0 g of NaOH dissolved in 1 kg of water results in a one-molal NaOH solution. If 20.0 g of NaOH, which is 0.500 mol of NaOH, is dissolved in exactly 1 kg of water, the concentration of the solution is 0.500 m NaOH.
How do you find the mass of NaCl?
What is the molar mass of 2NaCl?
The molar mass and molecular weight of 2NaCl is 116.8855.
What is a 1 mole?
A mole is defined as 6.02214076 × 1023 of some chemical unit, be it atoms, molecules, ions, or others. The mole is a convenient unit to use because of the great number of atoms, molecules, or others in any substance.
How much is a mole?
One mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). The number 6.022 × 10²³ is known as Avogadro’s number or Avogadro’s constant. The concept of the mole can be used to convert between mass and number of particles.. Created by Sal Khan.
How many moles are in 12g of H2?
How many grams H2 in 1 mol? The answer is 2.01588. We assume you are converting between grams H2 and mole.
What is the mass of 10 moles of Na2SO3?
Images related to the topicWhat is the mass of 10 moles of Na2SO3?

What is the mass in grams of 1.5 moles of sodium chloride?
1.5 Moles of Sodium = 34.484655 Grams
Please enter another number of moles of sodium that you want converted to grams.
How many moles are in h2o?
Water (H2O) is made from 2 atoms of hydrogen and 1 atom of oxygen. A mole of water molecules would be 2 moles of hydrogen atoms plus 1 mole of oxygen atoms. From the periodic table we see the atomic weight of hydrogen is 1.0079 and the atomic weight of oxygen is 15.9994.
Related searches to Which is the mass of 8 moles of NaCl?
- what is the mass of 4.50 moles of nacl
- what is the mass of 3.75 moles of nacl
- how many moles are in 3.6 grams of nacl
- how many molecules are in 3.6 g of nacl
- how many moles of atoms are in one mole of alpo4
- what is the mass in grams of 1 50 moles of sodium chloride nacl
- how many molecules are in 3 6 g of nacl
- how many grams are in 1 946 moles of nacl
- molar mass of nacl
- what is the mass of 2 moles of nacl
- 1 mole of nacl
- what is the mass in grams of 1 946 moles of nacl
- what is the mass in grams of 1.946 moles of nacl
- how many moles are in 3 6 grams of nacl
- what is the mass of 1.5 moles of nacl
- which is the mass of 8 moles of nacl
- how many grams are in 1.946 moles of nacl?
- what is the mass of 1.75 moles of nacl
Information related to the topic Which is the mass of 8 moles of NaCl?
Here are the search results of the thread Which is the mass of 8 moles of NaCl? from Bing. You can read more if you want.
You have just come across an article on the topic Which is the mass of 8 moles of NaCl?. If you found this article useful, please share it. Thank you very much.