Are you looking for an answer to the topic “Which Is The Strongest Electrolyte?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Yes, sodium chloride is indeed an electrolyte. In fact, common salt (NaCl) is a great example of a strong electrolyte. A strong electrolyte is an electrolyte that almost completely ionizes when dissolved in a polar solvent.Substances that completely dissociates into ions are called strong electrolytes. Example: Sodium chloride, potassium chloride, lead bromide, sodium hydroxide, potassium hydroxide, hydrochloric acid, nitric acid, sulfuric acid, etc.A strong electrolyte is a solution in which a large fraction of the dissolved solute exists as ions. Ionic compounds, and some polar compounds, are completely broken apart into ions and thus conduct a current very well—this makes them strong electrolytes.
- hydrochloric acid, HCl.
- hydroiodic acid, HI.
- hydrobromic acid, HBr.
- nitric acid, HNO3
- sulfuric acid, H2SO4
- chloric acid, HClO3
- perchloric acid, HClO4
| Strong Electrolytes | strong acids | HCl, HBr, HI, HNO3, HClO3, HClO4, and H2SO4 |
|---|---|---|
| strong bases | NaOH, KOH, LiOH, Ba(OH)2, and Ca(OH)2 | |
| salts | NaCl, KBr, MgCl2, and many, many more | |
| Weak Electrolytes | ||
| weak acids | HF, HC2H3O2 (acetic acid), H2CO3 (carbonic acid), H3PO4 (phosphoric acid), and many more |
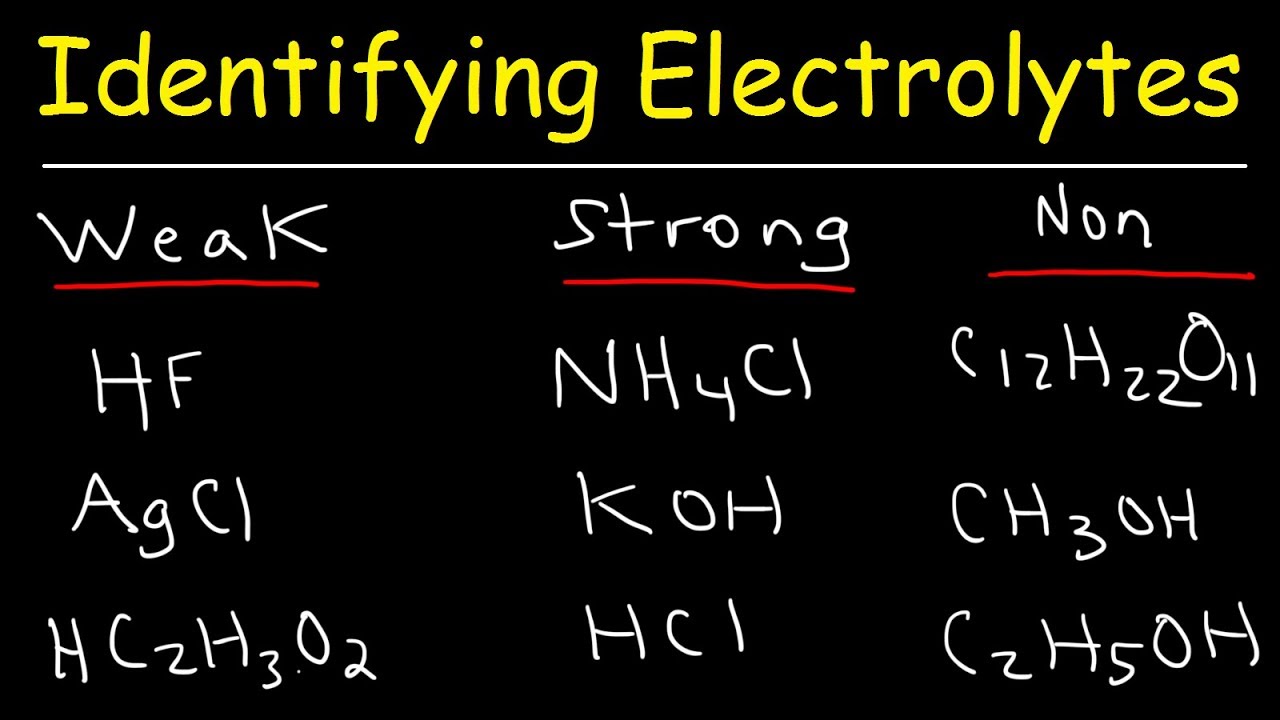
What are the 7 strong electrolytes?
- hydrochloric acid, HCl.
- hydroiodic acid, HI.
- hydrobromic acid, HBr.
- nitric acid, HNO3
- sulfuric acid, H2SO4
- chloric acid, HClO3
- perchloric acid, HClO4
Is NaCl the strongest electrolyte?
Yes, sodium chloride is indeed an electrolyte. In fact, common salt (NaCl) is a great example of a strong electrolyte. A strong electrolyte is an electrolyte that almost completely ionizes when dissolved in a polar solvent.
Identifying Strong Electrolytes, Weak Electrolytes, and Nonelectrolytes – Chemistry Examples
Images related to the topicIdentifying Strong Electrolytes, Weak Electrolytes, and Nonelectrolytes – Chemistry Examples

What is an example of a strong electrolyte?
Substances that completely dissociates into ions are called strong electrolytes. Example: Sodium chloride, potassium chloride, lead bromide, sodium hydroxide, potassium hydroxide, hydrochloric acid, nitric acid, sulfuric acid, etc.
What is a strong electrolyte in chemistry?
A strong electrolyte is a solution in which a large fraction of the dissolved solute exists as ions. Ionic compounds, and some polar compounds, are completely broken apart into ions and thus conduct a current very well—this makes them strong electrolytes.
What is strong and weak electrolyte?
Electrolytes are substances which, when dissolved in water, break up into cations (plus-charged ions) and anions (minus-charged ions). We say they ionize. Strong electrolytes ionize completely (100%), while weak electrolytes ionize only partially (usually on the order of 1–10%).
Is carbon a strong electrolyte?
…
Table of Strong Electrolytes, Weak Electrolytes, and Nonelectrolytes.
| Strong Electrolytes | |
|---|---|
| other carbon compounds | C5H12 (pentane) |
Is HF a strong electrolyte?
Therefore, HF is a weak electrolyte. Strong and weak electrolytes can also be applied to bases and other compounds that dissolve in water to produce ions.
See some more details on the topic Which Is The Strongest Electrolyte? here:
Strong electrolyte – Wikipedia
A strong electrolyte is a solution/solute that completely, or almost completely, ionizes or dissociates in a solution. These ions are good conductors of …
Strong Electrolyte Definition and Examples – ThoughtCo
HCl (hydrochloric acid), H2SO4 (sulfuric acid), NaOH (sodium hydroxide) and KOH (potassium hydroxide) are all strong electrolytes.
How to Find Out If a Compound Is a Strong Electrolyte
Examine whether the compound is a strong base. Strong bases are also strong electrolytes. Compounds that are formed with the hydroxide ion, OH-, …
Strong Electrolytes and Weak Electrolytes Chemistry Tutorial
Guidelines for Determining the Strength of an Electrolyte · Acids: Most acids are weak acids and therefore weak electrolytes. · Bases: Strong bases are strong …
Is CuSO4 a strong electrolyte?
Two compounds that are strong electrolytes are the ionic compounds ZnSO4 and CuSO4.
Is CCl4 a strong electrolyte?
No, CCl4 is not an electrolye. An electrolye is a material that causes ions (charged entities) to form in the solvent. Carbon tetrachloride neither dissociates into ions nor induces ion formation in the solvent. The reason is that the C−Cl bond is rather strong and won’t break under normal solution conditions.
Which of the two is strong electrolyte?
1 Answer. B is a strong electrolyte. The molar conductivity increases slowly with dilution as there is no increase in the number of ions on dilution as strong electrolytes are completely dissociated.
Which of the following is a strong electrolyte in solution?
A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in a solution. HCl (hydrochloric acid), H2SO4, NaOH and KOH are all strong electrolytes.
How to Identify Strong, Weak, and Non-Electrolytes Examples Practice Problems
Images related to the topicHow to Identify Strong, Weak, and Non-Electrolytes Examples Practice Problems

Which is not a strong electrolyte?
Weak Electrolyte Examples
HC2H3O2 (acetic acid), H2CO3 (carbonic acid), NH3 (ammonia), and H3PO4 (phosphoric acid) are all examples of weak electrolytes. Weak acids and weak bases are weak electrolytes. In contrast, strong acids, strong bases, and salts are strong electrolytes.
Is ammonia a strong electrolyte?
Thus, ammonia is a weak base, and like acetic acid, does not conduct electricity nearly as well as aqueous salt. So ammonia is a weak electrolyte as well.
Is glucose a strong electrolyte?
Is C6 H12 O6 (s) → H2O C6 H12 O6 (aq) a nonelectrolyte, weak electrolyte or a strong electrolyte? The substance above (glucose) is a nonelectrolyte. All nonelectrolytes do not conduct electricity when dissolved in water.
Is h2o a strong electrolyte?
By this definition, water can be considered a weak electrolyte. Water partially dissolves into positively charged hydrogen ions and negatively charged hydroxide (hydrogen and oxygen) ions, but most water molecules stay intact.
Is NaOH a strong electrolyte?
Strong acids, strong bases, and ionic salts that are not weak acids or bases are strong electrolytes. Salts much have high solubility in the solvent to act as strong electrolytes. HCl (hydrochloric acid), H2SO4 (sulfuric acid), NaOH (sodium hydroxide) and KOH (potassium hydroxide) are all strong electrolytes.
Is CaCl2 a strong electrolyte?
When calcium chloride dissolves in water it breaks up into its respective ions, ( charged particles) one mole of CaCl2 breaks up into one mole of a Ca+2 ion and 2 moles of Cl-1 ions thus it is a strong electrolyte and will conduct electricity when dissolved in water.
Is potassium iodide a strong electrolyte?
Hence, the potassium iodide is a strong electrolyte so, the ions will be completely dissociated into the water (aqueous solution).
Is CaCO3 a strong electrolyte?
Even insoluble ionic compounds (e.g., AgCl, PbSO4, CaCO3) are strong electrolytes, because the small amounts that do dissolve in water do so principally as ions; i.e., there is virtually no undissociated form of the compound in solution. 3.
Is methanol a strong electrolyte?
The answer is a non-electrolyte.
Methanol is a compound that is not capable of ionizing or dissociates into ions in a solution.
Is HNO3 a strong electrolyte?
Nitric acid, HNO3, is a common strong acid (“Common Strong Acids and Bases” table), and therefore is a strong electrolyte.
What Are Electrolytes?
Images related to the topicWhat Are Electrolytes?

Is NaNO3 a strong electrolyte?
…
Is NaNO3 an electrolyte when dissolved in water?
| Strong Electrolyte | Name |
|---|---|
| NaNO3 | Sodium Nitrate |
| NaCl | Sodium Chloride |
| LiCl | Lithium Chloride |
| KCl | Potassium Chloride |
Is HCN a strong electrolyte?
HCN is classified as a weak electrolyte.
This is because when dissolved in water, a low percentage of the HCN molecules ionize.
Related searches to Which Is The Strongest Electrolyte?
- based on table f which salt is the strongest electrolyte
- which one of the following substances is the strongest electrolyte
- which salt is the strongest electrolyte
- is ch3oh a strong electrolyte
- how to find the strongest electrolyte
- which one is the strongest electrolyte in the following
- which is the weakest electrolyte
- which is the strongest electrolyte
- which is the strongest electrolyte in the following
- which compound is most likely the strongest electrolyte
- how to know which is the strongest electrolyte
- strongest electrolyte list
- is koh a strong electrolyte
- are strong bases strong electrolytes
- is naoh a strong electrolyte
- weak electrolytes list
- what is the weakest electrolyte
- is acetic acid a strong electrolyte
- is hcl a strong electrolyte
- is nacl a strong electrolyte
Information related to the topic Which Is The Strongest Electrolyte?
Here are the search results of the thread Which Is The Strongest Electrolyte? from Bing. You can read more if you want.
You have just come across an article on the topic Which Is The Strongest Electrolyte?. If you found this article useful, please share it. Thank you very much.