Are you looking for an answer to the topic “Which isotope of carbon is used to determine the age of ancient remains?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
carbon-14 dating, also called radiocarbon dating, method of age determination that depends upon the decay to nitrogen of radiocarbon (carbon-14).Radiocarbon (C14 isotope) dating can be used to date artifacts as old as 50,000 years. This method is used because radiocarbon is a radioactive isotope of carbon.For nearly 70 years, archaeologists have been measuring carbon-14 levels to date sites and artifacts. Nothing good can last—and in the case of carbon-14, a radioactive isotope found in Earth’s atmosphere, that’s great news for archaeologists.
Which isotope of carbon is used to determine the age of ancient remains quizlet?
Radiocarbon (C14 isotope) dating can be used to date artifacts as old as 50,000 years. This method is used because radiocarbon is a radioactive isotope of carbon.
What carbon isotope is used in determining the age of archaeological material?
For nearly 70 years, archaeologists have been measuring carbon-14 levels to date sites and artifacts. Nothing good can last—and in the case of carbon-14, a radioactive isotope found in Earth’s atmosphere, that’s great news for archaeologists.
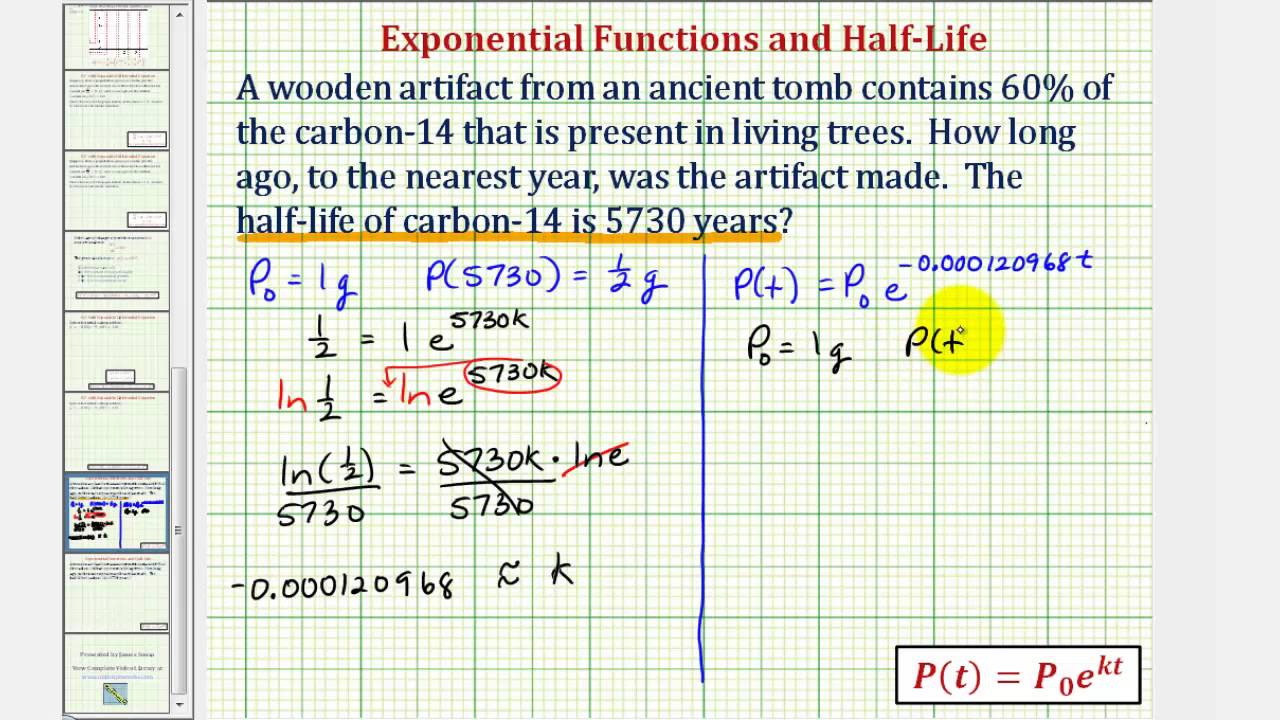
Ex: Exponential Model – Determine Age Using Carbon-14 Given Half Life
Images related to the topicEx: Exponential Model – Determine Age Using Carbon-14 Given Half Life

What isotope would you use to determine the age of ancient fossils?
The Carbon-14 Cycle. Radiocarbon dating (usually referred to simply as carbon-14 dating) is a radiometric dating method. It uses the naturally occurring radioisotope carbon-14 (14C) to estimate the age of carbon-bearing materials up to about 58,000 to 62,000 years old.
What is carbon-14 dating used for?
Radiocarbon dating (also referred to as carbon dating or carbon-14 dating) is a method for determining the age of an object containing organic material by using the properties of radiocarbon, a radioactive isotope of carbon.
What is the difference between carbon-12 and 14?
Atoms of carbon-12 have 6 neutrons, while atoms of carbon-14 contain 8 neutrons. A neutral atom would have the same number of protons and electrons, so a neutral atom of carbon-12 or carbon-14 would have 6 electrons.
How can an isotope like carbon-14 be used to date dead organisms quizlet?
Carbon-14 or C-14 because there are 14 particles in its nucleus (6 protons & 8 neutrons). carbon 14 has decayed so much that it cannot be measured. materials that are organic, cannot be used to date rocks. scientist use radioactive isotopes with long half-lives.
How can carbon-14 be used to determine age?
- Enter the percent of carbon-14 left in the sample, i.e., 92 in the first row.
- The half-life of carbon 14 is 5,730 years. …
- You will get the calculated time elapsed, i.e., 689 years in the third row, and the sample’s age, i.e., 690 (+/-5) years, as the final result.
See some more details on the topic Which isotope of carbon is used to determine the age of ancient remains? here:
Radiocarbon helps date ancient objects—but it’s not perfect
Carbon is made up of three isotopes. The most abundant, carbon-12, remains stable in the atmosphere. On the other hand, carbon-14 is …
Radiocarbon dating – Wikipedia
Radiocarbon dating is a method for determining the age of an object containing organic material by using the properties of radiocarbon, a radioactive …
How do scientists figure out how old things are? | Live Science
For older objects, scientists don’t use carbon-14 as a measure of age. Instead, they often look to radioactive isotopes of other elements …
Applying Carbon-14 Dating to Recent Human Remains
Archaeologists have long used carbon-14 dating (also known as radiocarbon dating) to estimate the age of certain objects.
How is carbon-14 used to date fossils?
The carbon-14 decays with its half-life of 5,700 years, while the amount of carbon-12 remains constant in the sample. By looking at the ratio of carbon-12 to carbon-14 in the sample and comparing it to the ratio in a living organism, it is possible to determine the age of a formerly living thing fairly precisely.
What is uranium 238 used to date?
Uranium 238 has a half life of 4.5 billion years. Uranium can be used to date the age of the earth. If 50% of pure uranium’ is left in a sample the sample is assumed to be 4.5 billion years old.
What element is used to determine the age of fossils?
Scientists use carbon dating when determining the age of fossils that are less than 60,000 years old, and that are composed of organic materials such as wood or leather.
How do we determine the age of fossils?
Relative dating is used to determine a fossils approximate age by comparing it to similar rocks and fossils of known ages. Absolute dating is used to determine a precise age of a fossil by using radiometric dating to measure the decay of isotopes, either within the fossil or more often the rocks associated with it.
Why is carbon-14 radioactive and carbon-12 not?
Carbon-12 is stable, meaning it never undergoes radioactive decay. Carbon-14 is unstable and undergoes radioactive decay with a half-life of about 5,730 years (meaning that half of the material will be gone after 5,730 years).
How Carbon Dating Works
Images related to the topicHow Carbon Dating Works

What is the use of carbon-12?
Carbon-12 is of particular importance in its use as the standard from which atomic masses of all nuclides are measured, thus, its atomic mass is exactly 12 daltons by definition.
What is sodium 24 used for?
Sodium 24 is used as an electrolyte tracer to follow the path sodium takes in a person’s body to see if their uptake levels are within normal ranges, while sodium 22 is used in nuclear medicine imaging for positron emission tomography. Sodium -24 can also be used in non-medical applications.
How was the half-life of carbon-14 determined?
The samples were then measured after a period of time to find the new levels of radioactivity. The loss of radioactivity was then extrapolated backwards to find the half life.
What are C12 C13 and C14 called?
THE DIFFERENT ATOMIC WEIGHT VARIANTS OF AN ELEMENT ARE CALLED THE ISOTOPES OF THAT ELEMENT. (For example C12, C13, and C14 are all isotopes of carbon, all have 6 protons but each has a different number of neutrons).
How are C12 and C13 different?
Carbon 12, 13 and 14 are carbon isotopes, meaning that they have additional neutrons: Carbon 12 has exactly 6 protons and 6 neutrons ( hence the 12 ) Carbon 13 has 6 protons and 7 neutrons.
What is the difference between carbon-14 and carbon 16?
Secondly, they have a different number of neutrons (Carbon -14 has a mass number of 14 and Carbon-16 has a mass number of 16). To find the number of neutrons, follow this formula: Neutrons = Mass number – Atomic number For Carbon-14, you take 14-6=8. Carbon-14 has 8 Neutrons. For Carbon-16, you take 16-6=10.
Which isotope would be most useful for dating some of Earth’s oldest rocks?
The half-life of 238U is 4.5 billion years, i.e., the time it takes for half of the parent isotope atoms to decay into the daughter isotope. This isotope of uranium, 238U, can be used for absolute dating the oldest materials found on Earth, and even meteorites and materials from the earliest events in our solar system.
How does carbon-14 dating determine the age of petrified wood quizlet?
How does carbon-14 dating determine the age of petrified wood? Fixed 14C in petrified wood decays, while 14C in living wood is replenished.
Which radioisotope is used to determine the age of once living organisms?
carbon-14 dating, also called radiocarbon dating, method of age determination that depends upon the decay to nitrogen of radiocarbon (carbon-14).
What is the formula for carbon-14 dating?
Carbon 14 is a common form of carbon which decays over time. The amount of Carbon 14 contained in a preserved plant is modeled by the equation f(t) = 10e^{-ct}.
Determining the Age of a Fossil Using Carbon-14
Images related to the topicDetermining the Age of a Fossil Using Carbon-14

Is carbon-14 dating reliable?
They have their work cut out for them, however, because radiocarbon (C-14) dating is one of the most reliable of all the radiometric dating methods.
How much carbon-14 is in a human?
Answer and Explanation: Scientists have discovered that around 23 percent of the human body is made up of the Carbon-14 isotope.
Related searches to Which isotope of carbon is used to determine the age of ancient remains?
- Carbon dating là gì
- carbon dating la gi
- Carbon-14
- absolute dating
- Density of carbon
- radiometric dating
- carbon dating the web
- potassium argon
- Absolute dating
- which isotope of carbon is used to determine the age of ancient remains
- density of carbon
- carbon 14
- Carbon dating the web
- geological time
- Radiometric dating
Information related to the topic Which isotope of carbon is used to determine the age of ancient remains?
Here are the search results of the thread Which isotope of carbon is used to determine the age of ancient remains? from Bing. You can read more if you want.
You have just come across an article on the topic Which isotope of carbon is used to determine the age of ancient remains?. If you found this article useful, please share it. Thank you very much.