Are you looking for an answer to the topic “Which isotope of silicon is least abundant?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Silicon has nine isotopes, with mass numbers from 25-33. Si (the most abundant isotope, at 92.23%), 29Si (4.67%), and 30Si (3.1%) are stable; 32Si is a radioactive isotope produced by argon decay.Silicon, atomic number 14, has 24 known isotopes with mass numbers ranging from 22 to 45. Silicon has three stable iso- topes: 28Si with 92.23 % abundance, 29Si with 4.67 % abun- dance, and 30Si with 3.1 % abundance.A sample of naturally occurring silicon consists Si-28 (amu = 27.9769), Si-29 (amu = 28.9765) and Si-30 (amu = 29.9738). If the atomic weight of silicon is 28.0855 and the natural abundance of Si-29 is 4.67%, what are the natural abundances of Si-28 and Si-30?
| PubChem CID | 16048635 |
|---|---|
| Molecular Weight | 29.9737701 |
| Dates | Modify 2022-05-21 Create 2007-04-30 |
| Description | Silicon-30 atom is the stable isotope of silicon with relative atomic mass 29.9737702. The least abundant (3.09 atom percent) isotope of naturally occurring silicon. ChEBI |
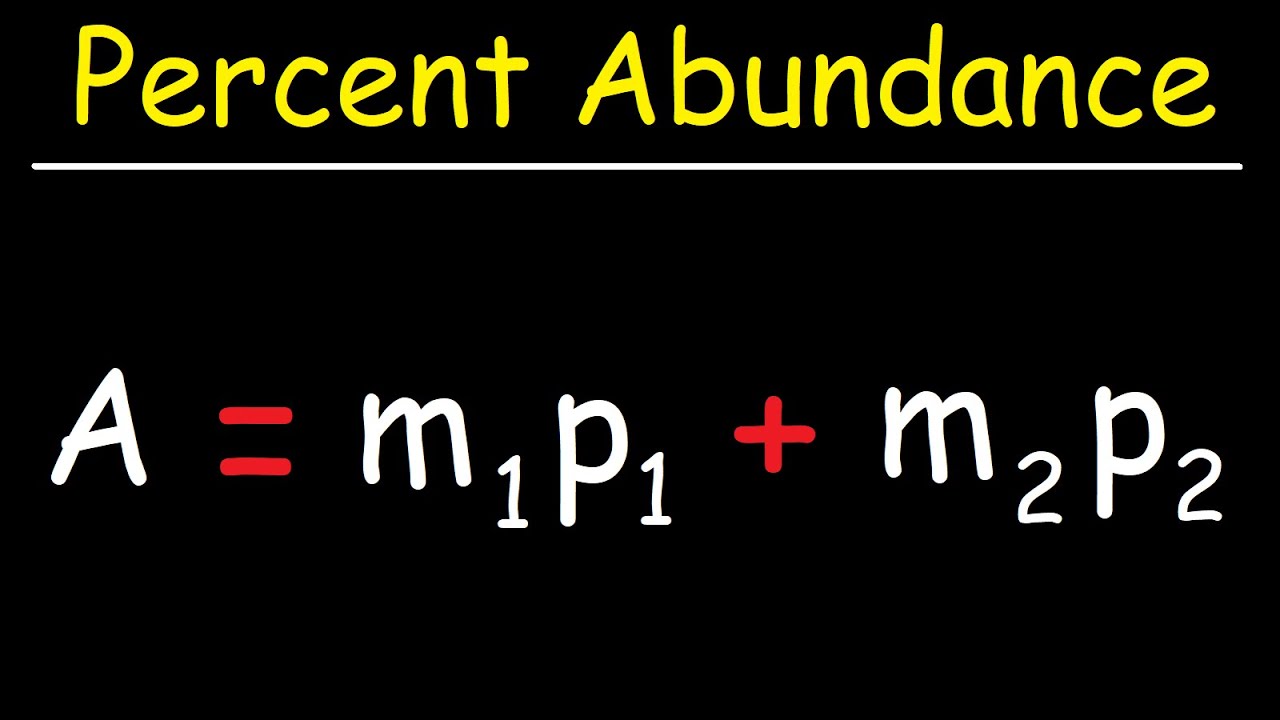
Which isotope of silicon is most abundant?
Silicon has nine isotopes, with mass numbers from 25-33. Si (the most abundant isotope, at 92.23%), 29Si (4.67%), and 30Si (3.1%) are stable; 32Si is a radioactive isotope produced by argon decay.
What is the abundance of isotopes of silicon?
Silicon, atomic number 14, has 24 known isotopes with mass numbers ranging from 22 to 45. Silicon has three stable iso- topes: 28Si with 92.23 % abundance, 29Si with 4.67 % abun- dance, and 30Si with 3.1 % abundance.
How To Find The Percent Abundance of Each Isotope – Chemistry
Images related to the topicHow To Find The Percent Abundance of Each Isotope – Chemistry

What is the percent abundance of silicon 29?
A sample of naturally occurring silicon consists Si-28 (amu = 27.9769), Si-29 (amu = 28.9765) and Si-30 (amu = 29.9738). If the atomic weight of silicon is 28.0855 and the natural abundance of Si-29 is 4.67%, what are the natural abundances of Si-28 and Si-30?
What is the natural abundance of silicon?
Natural abundance
Silicon makes up 27.7% of the Earth’s crust by mass and is the second most abundant element (oxygen is the first). It does not occur uncombined in nature but occurs chiefly as the oxide (silica) and as silicates.
How do you find the abundance of isotopes?
To calculate the percent abundance of each isotope in a sample of an element, chemists usually divide the number of atoms of a particular isotope by the total number of atoms of all isotopes of that element and then multiply the result by 100.
Which isotope has the highest isotopic abundance?
The most abundant isotope is Si-28 which accounts for 92.23% of naturally occurring silicon.
What are the three isotopes of silicon?
Silicon has three stable isotopes: 28Si, 29Si, and 30Si with respective abundances of 92.23%, 4.67%, and 3.1%. Si is a primary nucleus that exists owing to the nuclear oxygen-burning processes within stars whose initial compositions are hydrogen and helium rich, the two left elements from the Big Bang.
See some more details on the topic Which isotope of silicon is least abundant? here:
Isotopes of silicon – Wikipedia
Silicon (14Si) has 23 known isotopes, with mass numbers ranging from 22 to 44. … Si (the most abundant isotope, at 92.23%), 29Si (4.67%), and 30Si (3.1%) are …
Periodic Table–Silicon – USGS — Isotope Tracers — Resources
Silicon has nine isotopes, with mass numbers from 25-33. … Si (the most abundant isotope, at 92.23%), 29Si (4.67%), and 30Si (3.1%) are stable; 32Si is a …
Silicon Isotopes – an overview | ScienceDirect Topics
There are four isotopes of silicon (Si) exist in the natural environment—28Si, 29Si, 30Si and 32Si. The first three isotopes are stable isotopes and the last …
Silicon Isotopes | SpringerLink
Silicon has three stable isotopes: 28Si, 29Si, and 30Si with respective abundances of 92.23%, 4.67%, and 3.1%. … Si is a primary nucleus that exists owing to …
How do you find relative abundance?
- (M1)(x) + (M2)(1-x) = M(E)
- Example problem: If the masses of one isotope of nitrogen, nitrogen-14, is 14.003 amu and another isotope, nitrogen-15, is 15.000 amu, find the relative abundance of the isotopes.
- Use algebra to solve for x. …
- x = 0.996.
What is the percent abundance of an isotope?
Atoms that have the same number of protons but different numbers of neutrons are known as isotopes. Isotopes have different atomic masses. The relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element.
What is the average atomic mass of silicon given the following abundance information on the isotopes of silicon?
The average atomic mass of silicon is 28.086amu.
Physical Structure of Atoms: Calculate the Atomic Mass of Naturally Occurring Silicon
Images related to the topicPhysical Structure of Atoms: Calculate the Atomic Mass of Naturally Occurring Silicon

How many protons and how many neutrons are in the most abundant isotope of Si?
1 atom of silicon has 14 protons, 14 neutrons (in its most abundant isotope, silicon-14 ), and 14 electrons. 1 atom of oxygen has 8 protons, 8 neutrons (in its most stable isotope, oxygen-16 ), and 8 electrons.
How is Silicon 32 made?
The longest-lived radioisotope is 32Si, which is produced by cosmic ray spallation of argon. Its half-life has been determined to be approximately 150 years (with decay energy 0.21 MeV), and it decays by beta emission to 32P (which has a 14.28-day half-life) and then to 32S.
What percentage of silicon-28 isotope is present abundantly in the atmosphere?
The relative abundance of 28Si, 29Si and 30Si is 92.23%, 4.67% and 3.10%, respectively.
What are the name of the isotope in silicon?
isotopes of silicon are known: silicon-28, which makes up 92.21 percent of the element in nature; silicon-29, 4.70 percent; and silicon-30, 3.09 percent. Five radioactive isotopes are known.
Why is one isotope more abundant than another?
Although isotopes of the same element are twins when it comes to reactivity, the different number of neutrons means that they have a different mass. Certain isotopes are more abundant in some materials than others since some physical and chemical processes “prefer” one isotope over another.
How do you find the percent abundance of an isotope mass?
Step 1: List the known and unknown quantities and plan the problem. Change each percent abundance into decimal form by dividing by 100. Multiply this value by the atomic mass of that isotope. Add together for each isotope to get the average atomic mass.
How do you find the abundance of three isotopes?
Calculate the average atomic mass using the atomic masses of each isotope and their percent abundances. Divide each percent abundance by 100 to convert it to decimal form. Multiply this value by the isotope’s atomic mass. Add the atomic masses of each isotope together to get the average atomic mass.
What is the most abundant isotope of hydrogen?
Protium, 1H, has no neutrons in its nucleus and is the most common form of hydrogen, with an atomic mass of ~1.0078 Da (dalton) and an isotopic abundance of ~99.972% of all hydrogen on Earth.
Can you predict which isotope has a greater abundance based on its average atomic mass?
…
Using isotope abundance to calculate atomic weight.
| Isotope | Atomic Mass (amu) | Percent Abundance (%) |
|---|---|---|
| 26Mg | 25.982 | 11.17% |
Isotopes of Silicon Calculate Atomic Mass
Images related to the topicIsotopes of Silicon Calculate Atomic Mass

What does this say about the relative abundance of the three isotopes?
What does this say about the relative abundances of the three isotopes? the highest To abundance. Because the average atomic mass or is ü weighted average of the masses of each isotope, the AAM will be Closest to the isotope with the greatest abundance.
What is Silicon-32 used for?
Silicon-32, discovered in marine sponges, shows promise as a means for dating oceanographic phenomena. Silicon-32, discovered in marine sponges, shows promise as a means for dating oceanographic phenomena. Results.
Related searches to Which isotope of silicon is least abundant?
- silicon 28 mass number
- which isotope of silicon is least abundant in our atmosphere
- which isotope of silicon is least abundant in animal protein
- which isotope of silicon is most abundant
- silicon 28 protons neutrons electrons
- silicon-29 atomic number
- silicon 29 atomic mass
- silicon-28 protons neutrons electrons
- silicon 29 atomic number
- which isotope of silicon is least abundant in mediterranean diets
- silicon 32
- silicon isotopes natural abundance
- which isotope of silicon is least abundant in the universe
- silicon-32
- which isotope of silicon is least abundant in
- which isotope of silicon is least abundant in water
- how many isotopes does silicon have
- relative atomic mass of silicon 28 29 30
Information related to the topic Which isotope of silicon is least abundant?
Here are the search results of the thread Which isotope of silicon is least abundant? from Bing. You can read more if you want.
You have just come across an article on the topic Which isotope of silicon is least abundant?. If you found this article useful, please share it. Thank you very much.