Are you looking for an answer to the topic “Which kind of solvents are used in extraction?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Organic solvents with low polarity such as hexanes, toluene, dichloromethane and diethyl ether are usually chosen as the organic extracting solvent.The most common pair of extraction solvents used is diethyl ether (often referred to as simply ‘ether’) and water. Polarity is a relative term – ether is considered nonpolar and water polar. The fact that two phases are observed upon adding one to the other is a consequence of their different polarities.Methanol was identified as the most effective solvent for the extraction, resulting in the highest extraction yield (33.2%) as well as the highest content of phenolic (13.36 mg GAE/g DW), flavonoid (1.92 mg QE/g DW), alkaloid (1.40 mg AE/g DW), and terpenoids (1.25%, w/w).

What type of solvent is used in extraction?
The most common pair of extraction solvents used is diethyl ether (often referred to as simply ‘ether’) and water. Polarity is a relative term – ether is considered nonpolar and water polar. The fact that two phases are observed upon adding one to the other is a consequence of their different polarities.
What is the best solvent for extraction?
Methanol was identified as the most effective solvent for the extraction, resulting in the highest extraction yield (33.2%) as well as the highest content of phenolic (13.36 mg GAE/g DW), flavonoid (1.92 mg QE/g DW), alkaloid (1.40 mg AE/g DW), and terpenoids (1.25%, w/w).
Solvent used in Extraction Processes
Images related to the topicSolvent used in Extraction Processes

Why is diethyl ether used in extraction?
Diethyl ether is a common laboratory aprotic solvent. It has limited solubility in water (6.05 g/100 ml at 25 °C) and dissolves 1.5 g/100 g (1.0 g/100 ml) water at 25 °C. This, coupled with its high volatility, makes it ideal for use as the non-polar solvent in liquid-liquid extraction.
What are the different types of extracts?
- Liquid–liquid extraction.
- Solid-phase extraction.
- Acid-base extraction.
- Supercritical fluid extraction.
- Ultrasound-assisted extraction.
- Heat reflux extraction.
- Mechanochemical-assisted extraction.
- Maceration.
Why is methanol used in extraction?
The polarity of the solvent used will determine the kind of compounds that can be extracted. Most at times, methanol is a polar solvent used to extract bioactive compounds from extracts and has very good yields when compared to other solvents (methanol has better yields than ethanol).
Why ethanol is used in extraction?
Effective – Ethanol is widely used in oil extraction for its ability to extract a potent amount of oil. Ethanol is very effective in separating oils from the plant material, resulting in large extraction quantities.
What alcohol is used for extraction?
Typically, high-proof alcohols (190 and 200) have been used for extraction applications. Ethanol is emerging as one of the more popular solvents, because it is safe for infused edibles and compatible with any type of container. Ethanol also provides consistent results while being easily recoverable.
See some more details on the topic Which kind of solvents are used in extraction? here:
Extraction in Theory and Practice (Part I) – UCLA Chemistry …
Commonly used solvents like ethyl acetate (8.1 %), diethyl ether (6.9 %), dichloromethane (1.3 %) and chloroform (0.8 %) dissolved up to 10 …
Extraction
The three most common types of extractions are: liquid/liquid, liquid/solid, and acid/base (also known as a chemically active extraction). The coffee and tea …
How Does Solvent Extraction Work?
Popular solvents include water and organic compounds like benzene, tetrachloroethylene and turpentine. What Is Solvent Extraction? Solvent …
Liquid–liquid extraction – Wikipedia
Liquid–liquid extraction (LLE), also known as solvent extraction and partitioning, is a method to separate …
Why is NaOH used in extraction?
What do I use when to extract? In order to remove an acidic compound from a mixture, a base like NaOH or NaHCO3 is used.
Batch Extraction | Types Of Solvent Extraction | Solvent Extraction |
Images related to the topicBatch Extraction | Types Of Solvent Extraction | Solvent Extraction |
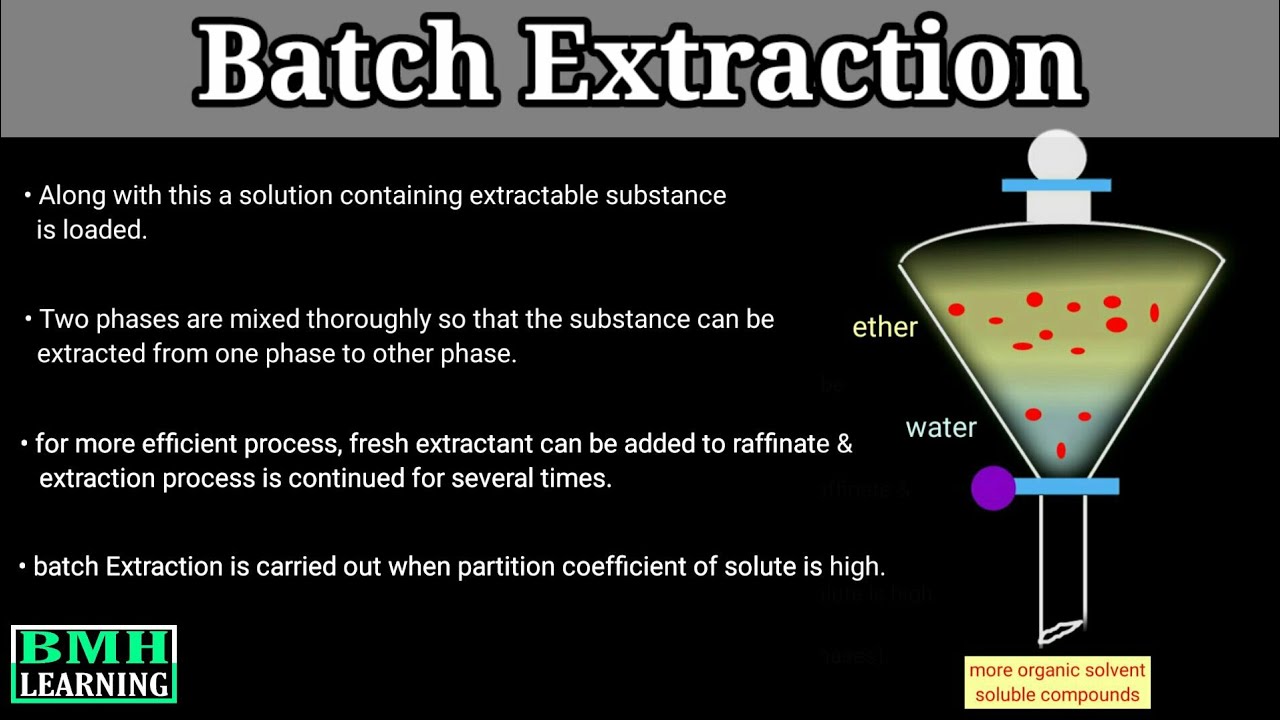
Why is dichloromethane used for extraction?
Dichloromethane is used as solvent in liquid-liquid extraction because caffeine has higher solubility in Dichloromethane as compared to other solvents. After separation of organic layer from the separating funnel it is then kept for evaporationso as to evaporate the dichloromethane present in it.
Why is ether used as solvent?
Ethers are generally considered as good solvents primarily because of its ability to accept H-bonds and combine with the London forces of the alkyl groups that are bonded to the oxygen. Ethers are regarded as excellent solvents for a wide range of organic compounds.
What are the 2 methods of extraction?
Extraction methods include solvent extraction, distillation method, pressing and sublimation according to the extraction principle. Solvent extraction is the most widely used method.
What is fluid extract?
A “fluid extract” means an alcoholic or aqueous-alcoholic liquid containing selected active components of herbal material and obtained by percolation or maceration of the herbal material in alcohol or a water-alcohol mixture. One part of the dry herbal material is used to make 1 part of the fluid extract.
What are two types of liquid, liquid extractions?
There are two types of extraction, liquid-liquid extraction also known as solvent extraction as well as solid-liquid extraction. Both extraction types are based on the same principle, the separation of compounds, based on their relative solubilities in two different immiscible liquids or solid matter compound.
Which is better ethanol or methanol?
The comparison between the decrease in HC emissions and the blended fuels indicates that methanol is more effective than ethanol. The lowest HC emissions are obtained with methanol-blended fuel (M50). When more combustion is complete, it will result in lower HC emissions.
Separating Components of a Mixture by Extraction
Images related to the topicSeparating Components of a Mixture by Extraction
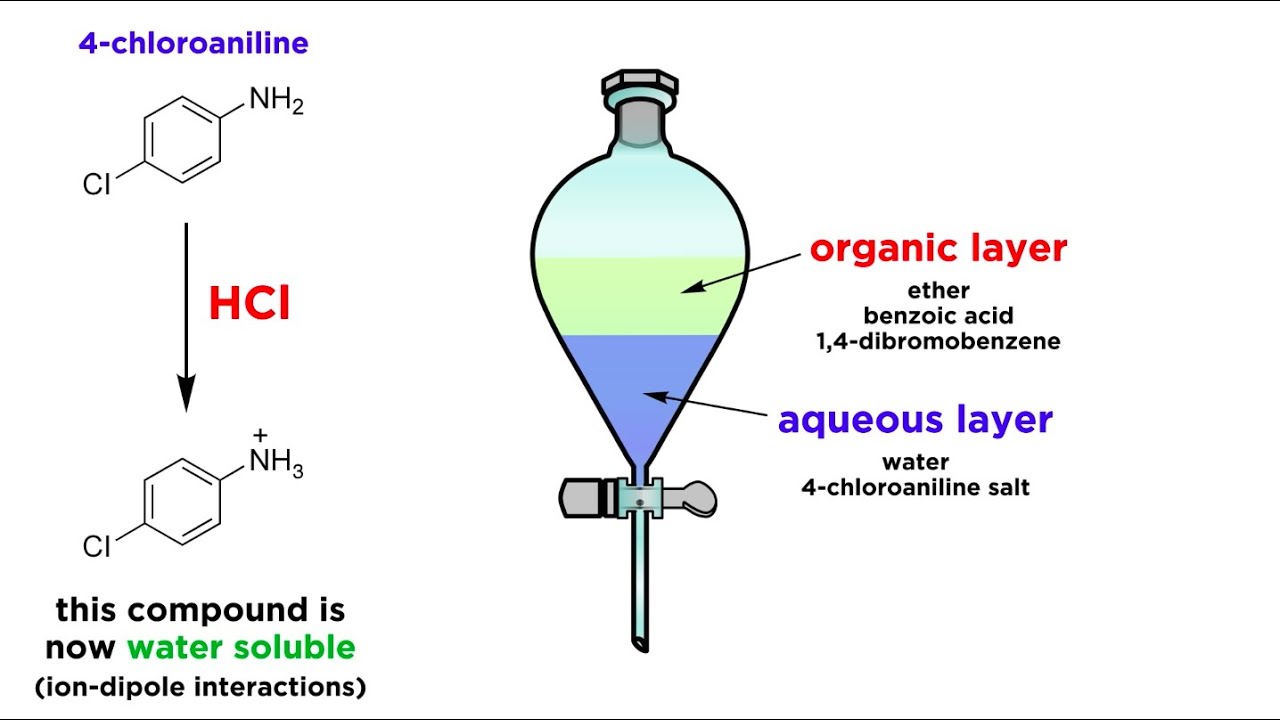
Why ethanol is used instead of methanol?
Ethanol is best one as it is safe though bit costly. Methanol is known poisonous though it evaporates but some very minute may remain in extract. Ethanol is better than methanol for same reasons.
What type of solvent is methanol?
Methanol is the primary alcohol that is the simplest aliphatic alcohol, comprising a methyl and an alcohol group. It has a role as an amphiprotic solvent, a fuel, a human metabolite, an Escherichia coli metabolite, a mouse metabolite and a Mycoplasma genitalium metabolite.
Related searches to Which kind of solvents are used in extraction?
- what are four characteristics of a good solvent for extraction
- disadvantages of solvent extraction
- what is the major disadvantage of using ether as an extraction solvent
- which kind of solvents are used in extraction of gold
- which kind of solvents are used in extraction workups
- which solvent is used in solvent extraction
- why is diethyl ether used in extraction
- which kind of solvents are used in extraction of dna
- why is dichloromethane a good solvent for extraction
- solvent extraction method examples
- which kind of solvents are used in extraction of caffeine
- types of solvent extraction
- what are four characteristics of a good solvent for extraction?
- which kind of solvents are used in extraction of oil
Information related to the topic Which kind of solvents are used in extraction?
Here are the search results of the thread Which kind of solvents are used in extraction? from Bing. You can read more if you want.
You have just come across an article on the topic Which kind of solvents are used in extraction?. If you found this article useful, please share it. Thank you very much.