Are you looking for an answer to the topic “Which law of thermodynamics states that absolute zero Cannot be reached?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
The third law of thermodynamics states, that the absolute zero-temperature 0 K cannot be reached by a finite number of steps. Therefore any technical reference to absolute zero is always referred to as an approximated value.Implications of the third law
Because a temperature of absolute zero is physically unattainable, the third law may be restated to apply to the real world as: The entropy of a perfect crystal approaches zero as its temperature approaches absolute zero.The third law of thermodynamics states, regarding the properties of closed systems in thermodynamic equilibrium: The entropy of a system approaches a constant value when its temperature approaches absolute zero.

What law is absolute zero Cannot be reached?
Implications of the third law
Because a temperature of absolute zero is physically unattainable, the third law may be restated to apply to the real world as: The entropy of a perfect crystal approaches zero as its temperature approaches absolute zero.
Which law of thermodynamics says we can approach but never reach absolute zero?
The third law of thermodynamics states, regarding the properties of closed systems in thermodynamic equilibrium: The entropy of a system approaches a constant value when its temperature approaches absolute zero.
Can Temperatures Go Below Absolute Zero?
Images related to the topicCan Temperatures Go Below Absolute Zero?

What is the 3rd law of thermodynamics in simple terms?
The third law of thermodynamic states that as the temperature of a system approaches absolute zero, its entropy becomes constant, or the change in entropy is zero. The third law of thermodynamics predicts the properties of a system and the behavior of entropy in a unique environment known as absolute temperature.
What does the second law of thermodynamics state?
The Second Law of Thermodynamics states that “in all energy exchanges, if no energy enters or leaves the system, the potential energy of the state will always be less than that of the initial state.” This is also commonly referred to as entropy.
What is the fourth law of thermodynamics?
Fourth law of thermodynamics’: the dissipative component of evolution is in a direction of steepest entropy ascent.
What is the First and Second Law of Thermodynamics?
“The first law of thermodynamics also known as the law of conservation of energy states that energy can neither be created nor destroyed, but it can be changed from one form to another.” “The second law of thermodynamics states that the entropy in an isolated system always increases.
What are the 2nd and 3rd laws of thermodynamics?
2nd Law of Thermodynamics – For a spontaneous process, the entropy of the universe increases. 3rd Law of Thermodynamics – A perfect crystal at zero Kelvin has zero entropy.
See some more details on the topic Which law of thermodynamics states that absolute zero Cannot be reached? here:
Which law states that absolute zero cannot be reached? a …
The third law of thermodynamics,the principle of temperature. This law states that the entropy at 0 is always equel to 0. … Still stuck? Get 1-on-1 help from an …
Laws of Thermodynamics | Physics Quiz – Quizizz
Which law of thermodynamics states that absolute zero cannot be reached? answer choices. 1st. 2nd. 3rd.
What is the third law of thermodynamics? | Live Science
The third law of thermodynamics states, “the entropy of a perfect crystal is zero when the temperature of the crystal is equal to absolute …
Third law of thermodynamics – Wikipedia
The third law of thermodynamics states, regarding the properties of closed systems in … In such a case, the entropy at absolute zero will be exactly zero.
Who proposed the third law of thermodynamics?
As per the third law of thermodynamics, the entropy of such a system is exactly zero. This law was developed by the German chemist Walther Nernst between the years 1906 and 1912.
Why is absolute zero not possible?
There’s a catch, though: absolute zero is impossible to reach. The reason has to do with the amount of work necessary to remove heat from a substance, which increases substantially the colder you try to go. To reach zero kelvins, you would require an infinite amount of work.
What is meant by Zeroth Law of Thermodynamics?
The zeroth law of thermodynamics states that if two bodies are each in thermal equilibrium with some third body, then they are also in equilibrium with each other.
What is Hess’s law used for?
Hess’s law can be used to calculate enthalpy changes that are difficult to measure directly. In this video, we’ll use Hess’s law to calculate the enthalpy change for the formation of methane, CH₄, from solid carbon and hydrogen gas, a reaction that occurs too slowly to be measured in the laboratory.
The Third Law of Thermodynamics: Absolute Zero
Images related to the topicThe Third Law of Thermodynamics: Absolute Zero
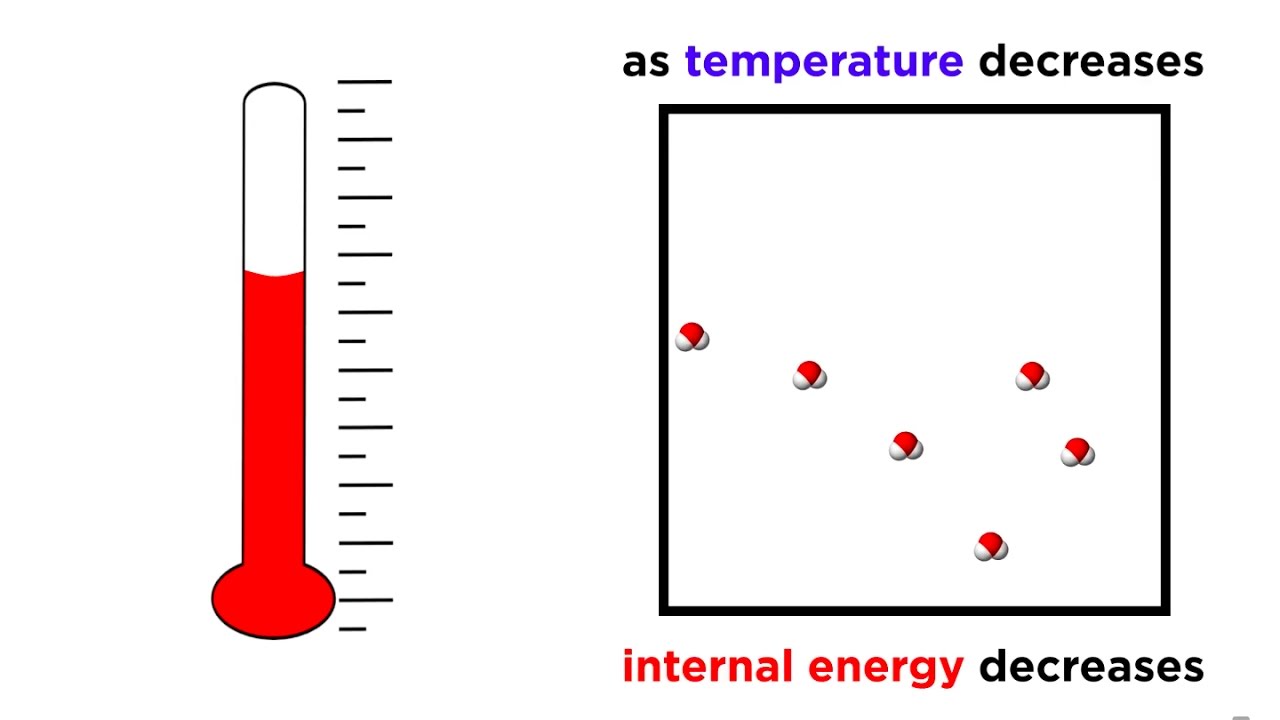
What is the Second Law of Thermodynamics in simple terms?
The second law of thermodynamics means hot things always cool unless you do something to stop them. It expresses a fundamental and simple truth about the universe: that disorder, characterised as a quantity known as entropy, always increases.
What does the 1st law of thermodynamics state?
The first law of thermodynamics states that energy can neither be created nor destroyed, only altered in form. For any system, energy transfer is associated with mass crossing the control boundary, external work, or heat transfer across the boundary. These produce a change of stored energy within the control volume.
What is the third law of thermodynamics quizlet?
Third Law of Thermodynamics. The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches zero. Absolute zero. The coldest temperature, 0 Kelvin, that can be reached. It is the hypothetical temperature at which all molecular motion stops.
What is entropy law?
Entropy is central to the second law of thermodynamics, which states that the entropy of isolated systems left to spontaneous evolution cannot decrease with time, as they always arrive at a state of thermodynamic equilibrium, where the entropy is highest.
What is the fifth law of thermodynamics?
The proposed Fifth Law is an extension of the Second Law of Thermodynamics. It postulates that, as a result of wear and tear, a machine will cease functioning as the sum of all the useful energy produced approaches the total energy expended in its construction.
What is an example of the 3rd Law of Thermodynamics?
State Third Law of Thermodynamics Example.
An example that states the third law of Thermodynamics is vapors of water are the gaseous forms of water at high temperatures. The molecules within the steam move randomly. Therefore, it has high Entropy.
Are there 3 or 4 laws of thermodynamics?
Traditionally, thermodynamics has recognized three fundamental laws, simply named by an ordinal identification, the first law, the second law, and the third law.
What is the 2nd Law of Thermodynamics and give an example?
Second law of thermodynamics:Statement,examples and applications. The second law of thermodynamics states that heat can flow spontaneously from a hot object to a cold object; heat will not flow spontaneously from a cold object to a hot object. Carnot engine, heat engine are some examples of second law of thermodynamics …
Which law of thermodynamics is entropy?
The second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous process; it never decreases.
What are the two main laws of thermodynamics?
The first law thermodynamics is also called the law of conservation of energy. According to the first law of thermodynamics, energy can neither be created nor destroyed in an isolated system. The second law of thermodynamics states that in an isolated system the entropy always increases.
Unattainability of absolute zero| Thermodynamics.
Images related to the topicUnattainability of absolute zero| Thermodynamics.

What are the limitations of first law of thermodynamics?
The limitation of the first law of thermodynamics is that it does not say anything about the direction of flow of heat. It does not say anything whether the process is a spontaneous process or not. The reverse process is not possible. In actual practice, the heat doesn’t convert completely into work.
What is an absolute zero temperature?
At zero kelvin (minus 273 degrees Celsius) the particles stop moving and all disorder disappears. Thus, nothing can be colder than absolute zero on the Kelvin scale.
Related searches to Which law of thermodynamics states that absolute zero Cannot be reached?
- which law of thermodynamics helps to explain how car engines work
- the second law of thermodynamics states that
- which law states can
- the first law of thermodynamics is basically the same as which law from physics 1
- First law of thermodynamics
- the first law of thermodynamics is basically the same as which law from physics 1?
- the first law of thermodynamics states that energy is
- first law of thermodynamics
- which law states “energy can neither be created nor destroyed. it can only be transformed”?
- which law of thermodynamics helps to explain how car engines work?
- zeroth law of thermodynamics
- which law of thermodynamics states that absolute zero cannot be reached quizizz
- which law of thermodynamics states that absolute zero cannot be reached
- Zeroth law of thermodynamics
Information related to the topic Which law of thermodynamics states that absolute zero Cannot be reached?
Here are the search results of the thread Which law of thermodynamics states that absolute zero Cannot be reached? from Bing. You can read more if you want.
You have just come across an article on the topic Which law of thermodynamics states that absolute zero Cannot be reached?. If you found this article useful, please share it. Thank you very much.