Are you looking for an answer to the topic “Which law of thermodynamics states that thermal energy Cannot be created or destroyed?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
1 First law of thermodynamics. The first law of thermodynamics is based on the law of conservation of energy, which states that energy cannot be created or destroyed, but can be transferred from one form to another.The Second Law of Thermodynamics states that “in all energy exchanges, if no energy enters or leaves the system, the potential energy of the state will always be less than that of the initial state.” This is also commonly referred to as entropy.The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero. The entropy of a system at absolute zero is typically zero, and in all cases is determined only by the number of different ground states it has.
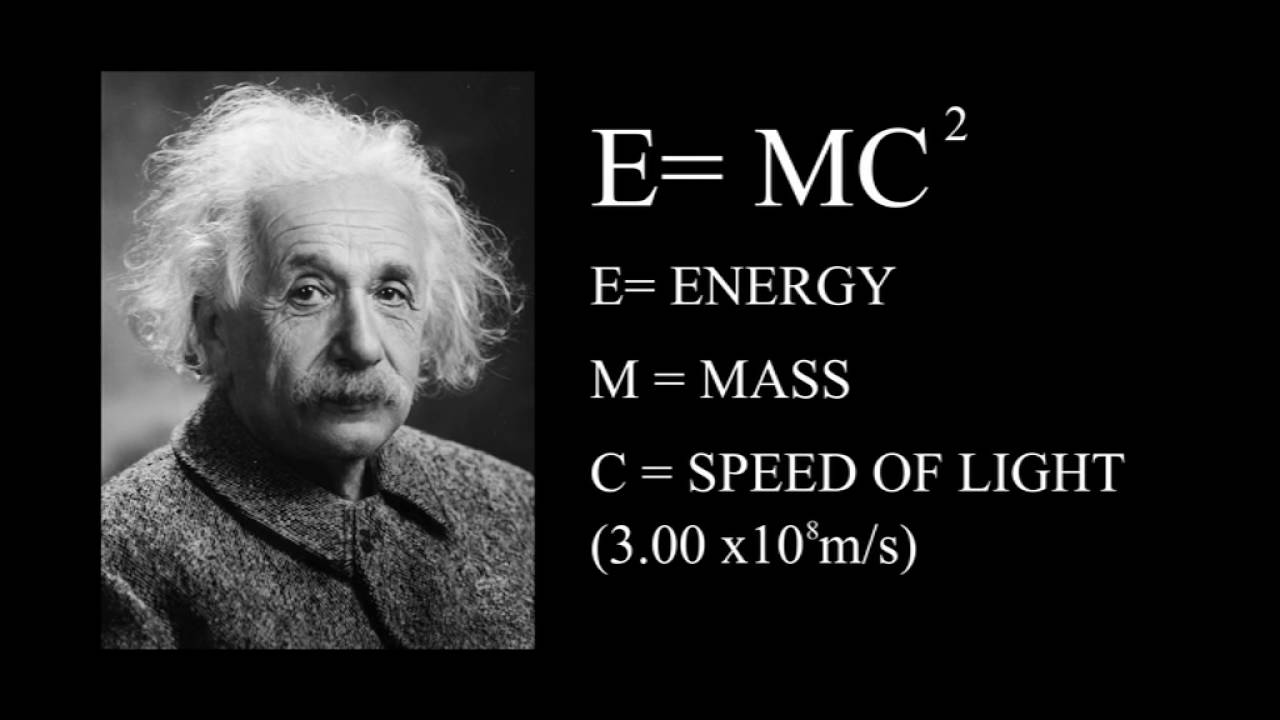
What does the 2nd law of thermodynamics state?
The Second Law of Thermodynamics states that “in all energy exchanges, if no energy enters or leaves the system, the potential energy of the state will always be less than that of the initial state.” This is also commonly referred to as entropy.
What does the 3rd law of thermodynamics state?
The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero. The entropy of a system at absolute zero is typically zero, and in all cases is determined only by the number of different ground states it has.
1st Law Thermodynamics
Images related to the topic1st Law Thermodynamics
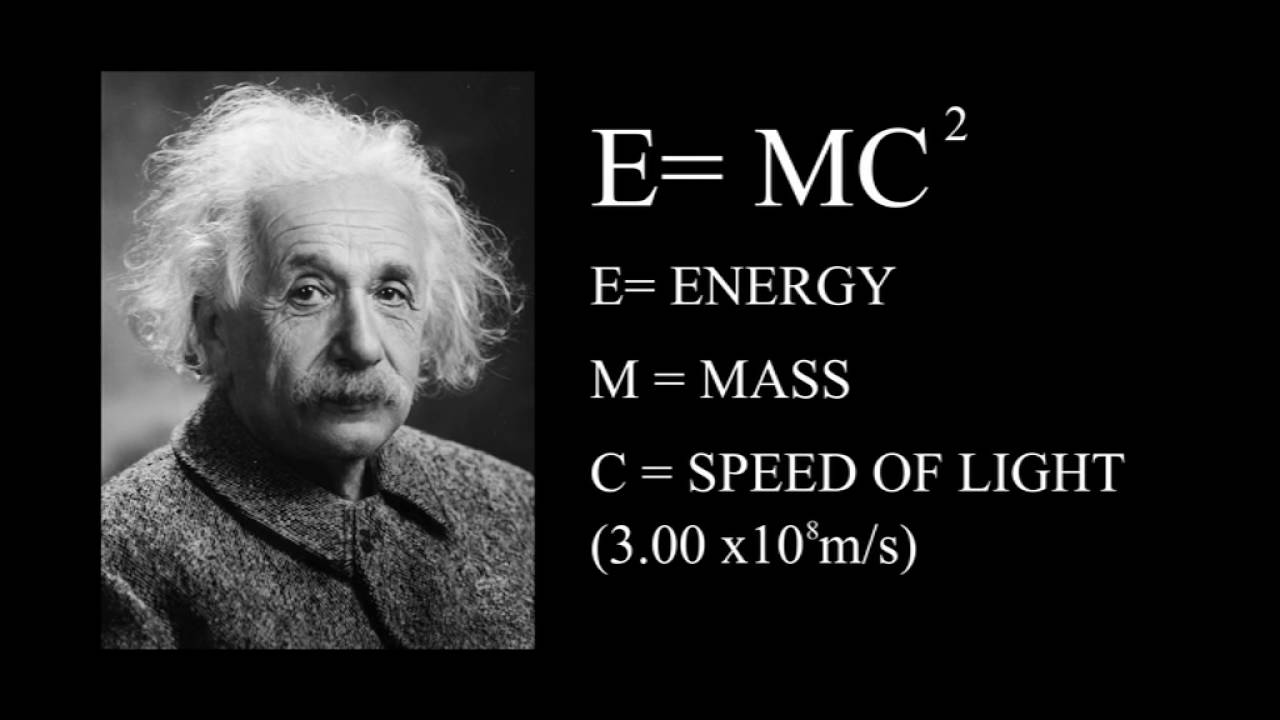
What are the 1st 2nd and 3rd laws of thermodynamics?
1st Law of Thermodynamics – Energy cannot be created or destroyed. 2nd Law of Thermodynamics – For a spontaneous process, the entropy of the universe increases. 3rd Law of Thermodynamics – A perfect crystal at zero Kelvin has zero entropy.
What is the 2nd law of thermodynamics in simple terms?
The second law of thermodynamics means hot things always cool unless you do something to stop them. It expresses a fundamental and simple truth about the universe: that disorder, characterised as a quantity known as entropy, always increases.
What does the 1st law of thermodynamics state?
The first law of thermodynamics states that energy can neither be created nor destroyed, only altered in form. For any system, energy transfer is associated with mass crossing the control boundary, external work, or heat transfer across the boundary. These produce a change of stored energy within the control volume.
What is the 4 law of thermodynamics?
Fourth law of thermodynamics’: the dissipative component of evolution is in a direction of steepest entropy ascent.
Why is it called the Zeroth Law of Thermodynamics?
The Zeroth law is called so because the first two laws of thermodynamics were established before, and they were named before. It was later found that zeroth law was more fundamental than the other laws of thermodynamics, that is the reason why it is called zeroth law of thermodynamics.
See some more details on the topic Which law of thermodynamics states that thermal energy Cannot be created or destroyed? here:
LAWS OF THERMODYNAMICS
The First Law of Thermodynamics (Conservation) states that energy is always conserved, it cannot be created or destroyed. In essence, energy can be converted …
The laws of thermodynamics (article) | Khan Academy
Put another way, the First Law of Thermodynamics states that energy cannot be created or destroyed. It can only change form or be transferred from one …
Laws of thermodynamics – Wikipedia
The first law of thermodynamics states that, when energy passes into or out of a system (as work, heat, or matter), the system’s internal energy changes in …
Law of conservation of energy
The law of conservation of energy states that energy can neither be created nor destroyed – only converted from one form of energy to …
What is entropy and third law of thermodynamics?
The third law of thermodynamics states that the entropy of a perfect crystal at a temperature of zero Kelvin (absolute zero) is equal to zero. Entropy, denoted by ‘S’, is a measure of the disorder/randomness in a closed system.
What is entropy law?
The second law of thermodynamics says, in simple terms, entropy always increases. This principle explains, for example, why you can’t unscramble an egg. The second law of thermodynamics states that processes that involve the transfer or conversion of heat energy are irreversible and always move toward more disorder. (
What does Zeroth Law of Thermodynamics states?
The zeroth law of thermodynamics states that if two bodies are each in thermal equilibrium with some third body, then they are also in equilibrium with each other.
What is the 3rd law of thermodynamics in simple terms?
In simple terms, the third law states that the entropy of a perfect crystal of a pure substance approaches zero as the temperature approaches zero. The alignment of a perfect crystal leaves no ambiguity as to the location and orientation of each part of the crystal.
What is the zeroth first and second law of thermodynamics?
→ The laws of thermodynamics are: First law of thermodynamics: Energy can neither be created nor be destroyed, it can only be transferred from one form to another. Second law of thermodynamics: The entropy of any isolated system always increases.
First Law of Thermodynamics, Basic Introduction – Internal Energy, Heat and Work – Chemistry
Images related to the topicFirst Law of Thermodynamics, Basic Introduction – Internal Energy, Heat and Work – Chemistry

What is the 1st and 2nd law of thermodynamics?
“The first law of thermodynamics also known as the law of conservation of energy states that energy can neither be created nor destroyed, but it can be changed from one form to another.” “The second law of thermodynamics states that the entropy in an isolated system always increases.
What is entropy 2nd law of thermodynamics?
The second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous process; it never decreases.
What does the 2nd law?
The second law states that the acceleration of an object is dependent upon two variables – the net force acting upon the object and the mass of the object. The acceleration of an object depends directly upon the net force acting upon the object, and inversely upon the mass of the object.
What is the 2nd law of thermodynamics and give an example?
Second law of thermodynamics:Statement,examples and applications. The second law of thermodynamics states that heat can flow spontaneously from a hot object to a cold object; heat will not flow spontaneously from a cold object to a hot object. Carnot engine, heat engine are some examples of second law of thermodynamics …
What is Delta H thermodynamics?
ΔH is the “enthalpy change“, Hf is the final enthalpy of the system (in a chemical reaction, the enthalpy of the products or the system at equilibrium), Hi is the initial enthalpy of the system (in a chemical reaction, the enthalpy of the reactants).
Who gave second law of thermodynamics?
Around 1850 Rudolf Clausius and William Thomson (Kelvin) stated both the First Law – that total energy is conserved – and the Second Law of Thermodynamics.
What is the fifth law of thermodynamics?
The proposed Fifth Law is an extension of the Second Law of Thermodynamics. It postulates that, as a result of wear and tear, a machine will cease functioning as the sum of all the useful energy produced approaches the total energy expended in its construction.
When was the Zeroth Law of Thermodynamics discovered?
This might also be expressed by saying that there is precisely one kind of non-mechanical, non-matter-transferring contact equilibrium between thermodynamic systems. According to Sommerfeld, Fowler coined the term zeroth law of thermodynamics while discussing the 1935 text by Meghnad Saha and B.N. Srivastava.
What are the laws of thermodynamics states?
Key Points. The first law, also known as Law of Conservation of Energy, states that energy cannot be created or destroyed in an isolated system. The second law of thermodynamics states that the entropy of any isolated system always increases.
What is reversible and irreversible process thermodynamics?
In terms of thermodynamics, a reversible process is where the participants go back to its initial form by inculcating minor or negligible changes in their surroundings. Contrarily, an irreversible process is a naturally occurring phenomenon, which does not go back to its original state.
EMM3213 | The Fundamental Principle of First and Second Law of Thermodynamics
Images related to the topicEMM3213 | The Fundamental Principle of First and Second Law of Thermodynamics

What is zeroth law of thermodynamics Shaalaa?
Solution. The zeroth law of thermodynamics states that if two systems, A and B, are in thermal equilibrium with a third system, C, then A and B are in thermal equilibrium with each other.
How many laws of thermodynamics are there?
The four laws of thermodynamics
The fundamental principles of thermodynamics were originally expressed in three laws.
Related searches to Which law of thermodynamics states that thermal energy Cannot be created or destroyed?
- the second law of thermodynamics states that quizlet
- laws of thermodynamics
- second law of thermodynamics
- energy cannot be created or destroyed which law
- energy cannot be created or destroyed who said
- the first law of thermodynamics states that
- zeroth law of thermodynamics
- third law of thermodynamics
Information related to the topic Which law of thermodynamics states that thermal energy Cannot be created or destroyed?
Here are the search results of the thread Which law of thermodynamics states that thermal energy Cannot be created or destroyed? from Bing. You can read more if you want.
You have just come across an article on the topic Which law of thermodynamics states that thermal energy Cannot be created or destroyed?. If you found this article useful, please share it. Thank you very much.