Are you looking for an answer to the topic “Which of the following condition is not correct for resonating structure?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
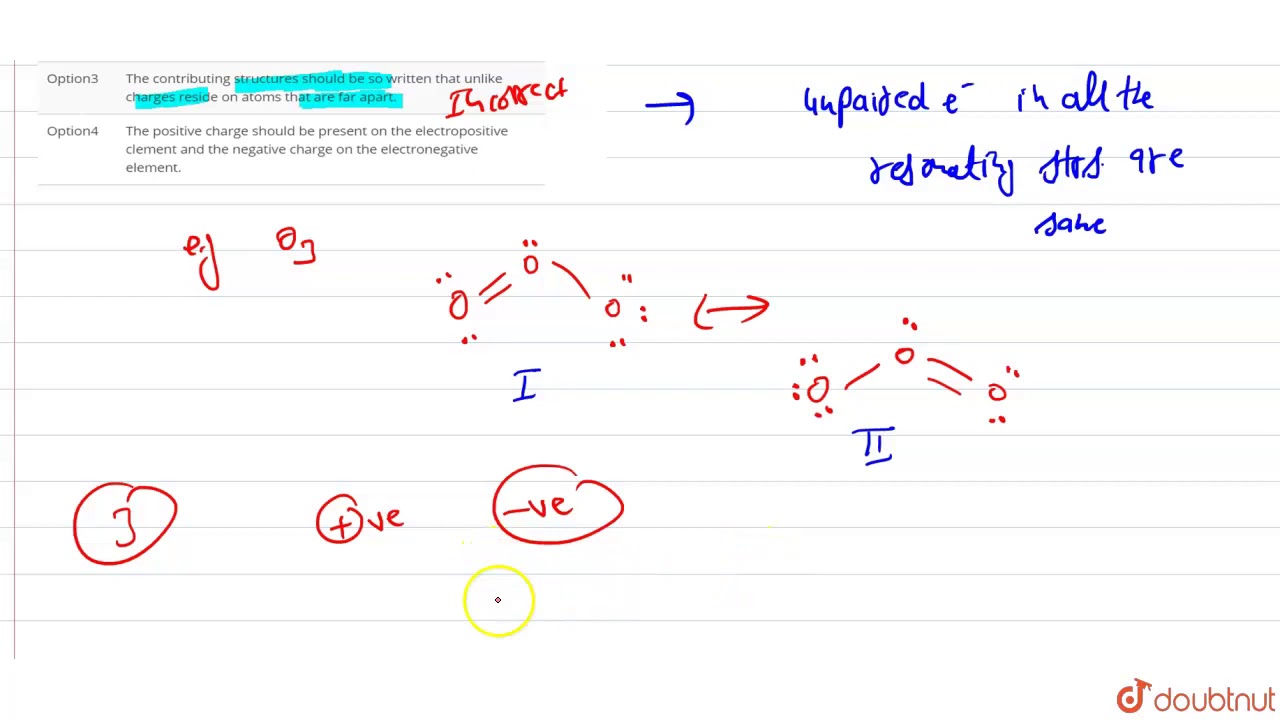
1 Answer. Correct option (c) The contributing structures should be so written that unlike charges reside on atoms that are far apart. Explanation: There is no restriction that resonating structures should have +ve and -ve charges on atoms that are far apart.Answer: DSolution: is not resonating structure of others.All the resonating structures should have the same number of unpaired electrons and the resonating structures should have the nearly same energies. In the resonating structure, the more electronegative atom has a negative charge and the more electropositive atom have a positive charge.
Which of the following is not resonating structure?
Answer: DSolution: is not resonating structure of others.
Which of the following conditions apply to resonating structure?
All the resonating structures should have the same number of unpaired electrons and the resonating structures should have the nearly same energies. In the resonating structure, the more electronegative atom has a negative charge and the more electropositive atom have a positive charge.
Which of the following conditions is not correct for resonating structures?
Images related to the topicWhich of the following conditions is not correct for resonating structures?

Which is incorrect regarding resonance structure?
In resonance structures, there should not be an equal number of electron pairs. Hence the statement A, B and D are correct. We know that the resonance structure should have the same number of electron pairs. Thus the incorrect statement about the resonance structure is C.
Which of the following pairs do not constitute resonating structure?
Solution : (a) In resonance, the position of atoms or nuclei remains fixed, so pairs in (ii) constitude resonance. In othe pairs, the position of atoms are changing , therefore, (i),(iii), and (iv) pairs do not constitude resonance structures.
Which of the following is not the correct resonance structure of methyl cyanate?
The option (C) represents incorrect resonance structure of methyl isocyanate. Note: Resonance structures are obtained by movement of pi electrons or unshared electrons. Electrons of sigma bonds do not move. Similarly, the atoms do not move and their position remains fixed.
Which of the following is not resonance stabilized?
Answer: CSolution: Lone pair will not participate in resonance because of berdt’s rule .
Do resonating structures have same number of unpaired electrons?
Resonance structures must have the same number of paired or unpaired electrons, but not necessarily the same number of bonds.
See some more details on the topic Which of the following condition is not correct for resonating structure? here:
Which of the following conditions is not correct for resonating …
1. The relative positions of all the atoms to be used in the structure must be the same. 2. The number of unpaired and paired electrons in the resonating forms …
Question 106, 2. Chemical Bonding and Nuclear Structure …
Therefore , the condition that is not correct for resonating structures is the contributing structures should be so written that unlike charges reside on atoms …
Do resonating structures have a real existence?
The canonical structures used to represent resonance have no real existence also. They are only for represenational purposes.
What does it mean when a molecule is resonant?
In chemistry terms, resonance describes the fact that electrons are delocalized, or flow freely through the molecule, which allows multiple structures to be possible for a given molecule.
Which of the following is correct regarding resonance?
1 Answer. Statement (c) is correct for resonance because resonance affects bond lengths but not bond angles while (a), (b) and (d) are incorrect statements.
Which statement is false regarding resonance?
Explanation: Resonance forms are hybrid in nature and hence it is not correct to say that they are in equilibrium with each other. 2. Identify the false statement regarding resonance. Explanation: Greater the number of charges, less stable and less significant gets the resonance form.
Which statement is true about resonance structures?
The answer is (c) Resonance structure illustrates that molecules are not a single structure and in fact, the molecule may equilibrate between multiple…
Which of the following conditions does not apply to resonating structures? | 11 | CHEMICAL BONDI…
Images related to the topicWhich of the following conditions does not apply to resonating structures? | 11 | CHEMICAL BONDI…

Which of the following pairs represent resonating structure?
R−C+=OandR−C≡O+
Was this answer helpful?
Which of the following is least stable resonating structure?
Structure (A) is the least stable resonating structure because in this structure the negative charge is present on the carbon atom attached −NH2 group which is electron donating in nature which will destabilize the molecule.
Which of the following is most stable resonating structure?
- ↑
- ∴ octet complete.
- ∴ most stable.
Which of these molecules is not resonance form of?
NH3 does not show any resonating structure due to the absence of double bond.
Which of the following resonance structure contributes the most of the resonance hybrid?
In a resonance hybrid, the bond lengths are always different from that of the contributing structure. The structures in which the negative charge resides on the most electronegative atoms and positive charge resides on the most electropositive structure, is most contributing.
Which of the following has two equivalent resonating structures?
The anion HCOO^- has two equivalent resonating structures.
Which of the following species can not show resonance?
C6H5NH3+ do not exhibit resonance effect as it do not have lone pair of electron to participate in resonance effect instead it has positive charge. Hence, option B is correct.
Which stability order is correct?
I > III > II.
Which of the following compounds is not resonance Stabilised ABCD?
Solution : Lone pair will not participate in resonance because of bredt.
What is hyper conjugation effect?
Hyperconjugation effect is a permanent effect in which localization of σ electrons of C-H bond of an alkyl group directly attached to an atom of the unsaturated system or to an atom with an unshared p orbital takes place.
Resonance Structures
Images related to the topicResonance Structures

What is the meaning of Hyperconjugation?
In organic chemistry, hyperconjugation (σ-conjugation or no-bond resonance) refers to the delocalization of electrons with the participation of bonds of primarily σ-character.
What are Mesomeric molecules?
The mesomeric effect in chemistry is a property of substituents or functional groups in a chemical compound. It is defined as the polarity produced in the molecule by the interaction of two pi bonds or between a pi bond and lone pair of electrons present on an adjacent atom.
Related searches to Which of the following condition is not correct for resonating structure?
- resonating structure are in the position of atoms
- which of the following resonating structure is not correct for co2
- resonating structures of molecules have
- which of the following is incorrect about hybridization
- incorrect resonating structure among the following is
- which of the following molecules have distorted geometry
- which of the following condition is not correct for resonating structure
- which of the following resonating structure is not correct for no3
- which of the following cannot be represented by resonance structures
Information related to the topic Which of the following condition is not correct for resonating structure?
Here are the search results of the thread Which of the following condition is not correct for resonating structure? from Bing. You can read more if you want.
You have just come across an article on the topic Which of the following condition is not correct for resonating structure?. If you found this article useful, please share it. Thank you very much.