Are you looking for an answer to the topic “Which of the following has highest electron affinity F minus CL minus?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
F− ions has the highest electron affinity (EA). F− ions are smaller in size than Cl− ions. When an electron is added to F−, the inter electronic repulsion is higher than when an electron is added to Cl− ions. Thus more energy is required to add electron to F− ion than to Cl− ion.Electron affinity of Cl is greater than that of F.Cl has higher affinity thanF because it can accommodate the incoming electron more easily thanF. F has small size and there will be repulsions for incoming electrons.
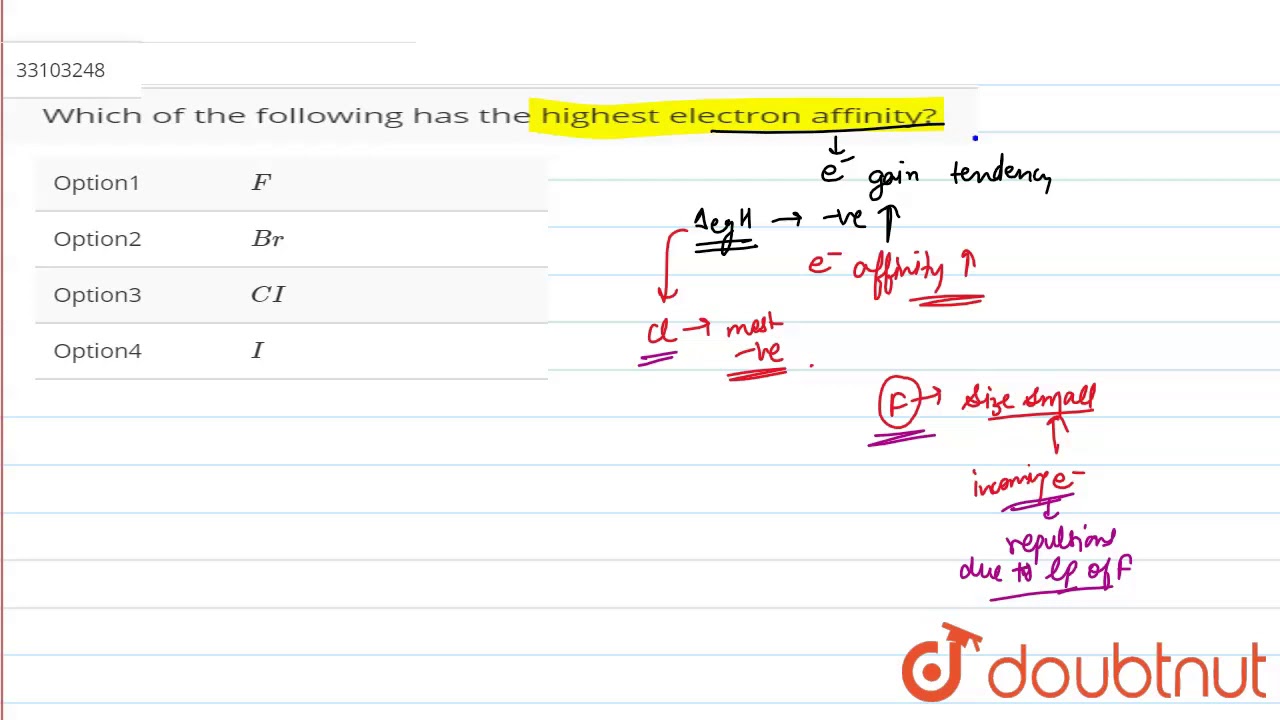
Which has more electron affinity F minus or Cl minus?
Electron affinity of Cl is greater than that of F.
Which of the following has highest electron affinity F or Cl?
Cl has higher affinity thanF because it can accommodate the incoming electron more easily thanF. F has small size and there will be repulsions for incoming electrons.
Which of the following has the highest electron affinity?
Images related to the topicWhich of the following has the highest electron affinity?

Which of the following has highest electron affinity F minus minus in?
Solution : Halongens have the highest electrons affinity .
Which out of Cl and Cl − has higher electron affinity?
Answer: Na+ has a higher electron affinity than Cl. The energy released when an electron is added to a neutral gaseous atom is known as electron affinity. The electron affinity increases across a period while it decreases down a group.
Which electron has highest affinity?
Halogens has higher electron affinity and it is supposed to be for fluorine, but chlorine has higher electron affinity than fluorine due to fluorine’s smaller size. Hence, among given options chlorine has highest electron affinity.
Which has maximum electron affinity?
Chlorine has the highest electron affinity among the elements. Its high affinity can be attributed to its large atomic radius, or size. Because chlorine’s outermost orbital is 3p, its electrons have a large amount of space to share with an incoming electron.
Which of the following has highest EA a F (-) B Cl (-) C Li (+) d Na (+)?
F− ions has the highest electron affinity (EA). F− ions are smaller in size than Cl− ions.
See some more details on the topic Which of the following has highest electron affinity F minus CL minus? here:
Which of the following has the highest electron affinity? a. F b …
Answer to: Which of the following has the highest electron affinity? a. F b. Cl c. Br In the periodic table, electron affinity decreases down in a group as.
Periodic Trends — Electron Affinity
The halogens in Group 7A all have large negative electron affinities, since they are only one electron away from having a noble gas configuration, they easily …
What Is Electron Affinity? | Trends & Chart | ChemTalk
Chlorine has the highest electron affinity among the elements. Its high affinity can be attributed to its large atomic radius, or size. Because …
Why Does Chlorine Have the Highest Electron Affinity?
Therefore, chlorine has a higher electron affinity than fluorine, and this orbital structure causes it to have the highest electron affinity of all of the …
know Electron affinity in just 4 minutes..why E.A of cl is greater than F?( for 11th,12th and bsc)..
Images related to the topicknow Electron affinity in just 4 minutes..why E.A of cl is greater than F?( for 11th,12th and bsc)..

Which of the following has the highest electron affinity 1 F (-) 2 O (-) 3 O 4 Na?
Fluorine has highest electron affinity in the periodic table.
What is the electron affinity of Cl?
For example, when a neutral chlorine atom in the gaseous form picks up an electron to form a Cl- ion, it releases an energy of 349 kJ/mol or 3.6 eV/atom. It is said to have an electron affinity of -349 kJ/mol and this large number indicates that it forms a stable negative ion.
Why is the electron affinity of fluorine less than chlorine?
Solution : Electron affinity of fluorine is less than that of chlorine because the atomic size of fluorine is very less than chlorine as a results there is a large electronic repulsion between the electrons of fluorine.
Which of the following has the highest electron affinity F Cl Br I?
Hence, chlorine has the highest electron affinity than fluorine and bromine.
Why chlorine has highest electron affinity than fluorine?
the electron affinity of the fluorine is less than chlorine because the size of fluorine is too small as size decreases from left to right inside period, whereas chlorine has a larger size to accommodate electrons hence electron affinity of chlorine is more than fluorine.
Which element has the highest electron affinity most negative value )?
Electron affinity of non metals is highest negative. Here, apart from Br all are metals. So, Br has most negative electron affinity.
Which element has highest Electron Affinity ?
Images related to the topicWhich element has highest Electron Affinity ?

Which of the following element has highest electron affinity A IB Br C Cl D F?
Chlorine has maximum electron affinity.
Which halogen has the highest electron affinity?
Therefore, chlorine has a higher electron affinity than fluorine, and this orbital structure causes it to have the highest electron affinity of all of the elements.
Related searches to Which of the following has highest electron affinity F minus CL minus?
- highest electron affinity is shown by
- which of the following elements has the greatest electron affinity ti cu zn kr
- which of the following has the least electronegativity
- which of the following elements has the greatest electron affinity
- which of the following elements has the least capability of attracting electrons
- which of the following elements has the greatest electron affinity? ti cu zn kr
- which of the following elements has the least capability of attracting electrons?
- which of the following elements has the greatest electron affinity ra ba sr ca
- which one has highest electron affinity
- which group has the highest electron affinity within a period
Information related to the topic Which of the following has highest electron affinity F minus CL minus?
Here are the search results of the thread Which of the following has highest electron affinity F minus CL minus? from Bing. You can read more if you want.
You have just come across an article on the topic Which of the following has highest electron affinity F minus CL minus?. If you found this article useful, please share it. Thank you very much.