Are you looking for an answer to the topic “Which of the following ion has highest electron affinity?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
F− ions has the highest electron affinity (EA). F− ions are smaller in size than Cl− ions. When an electron is added to F−, the inter electronic repulsion is higher than when an electron is added to Cl− ions. Thus more energy is required to add electron to F− ion than to Cl− ion.Electron affinity of Cl is greater than that of F.Therefore, the “greater” electron affinity (really, the more favorable electron affinity) is with O .
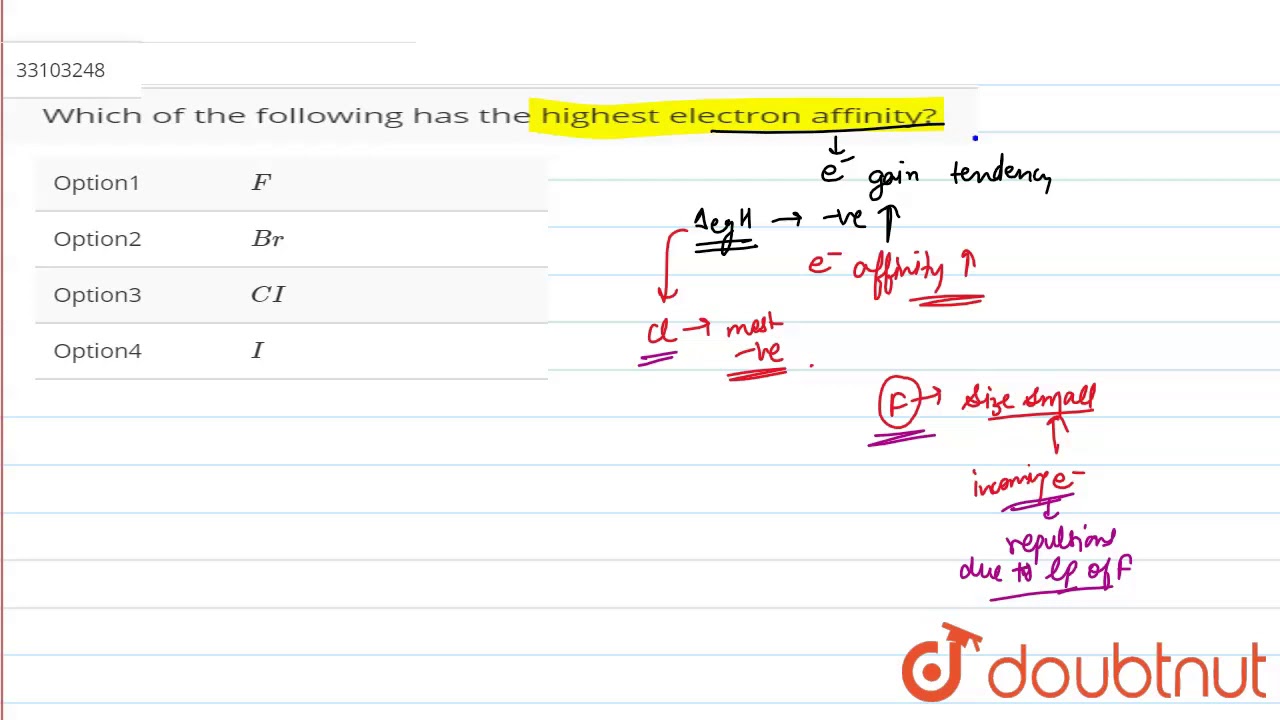
Table of Contents
Which has greater electron affinity F+ or Cl+?
Electron affinity of Cl is greater than that of F.
Which has higher electron affinity O or O?
Therefore, the “greater” electron affinity (really, the more favorable electron affinity) is with O .
Which of the following has the highest electron affinity?
Images related to the topicWhich of the following has the highest electron affinity?

Where is electron affinity the highest?
Electron affinity increases towards the top of the periodic table and towards the right. Thus, the top right elements (except for the noble gases) have the highest electron affinities – the greatest energy released when an electron is added.
Which of the following species has highest electron affinity 1 Li 2 O 3 O 4 N?
Solution : Halongens have the highest electrons affinity .
Whose electron affinity is more among F and Cl and why?
Electronegativity of fluorine is greater than that of chlorine but electron affinity of chlorine is greater than that of fluorine.
Which has more electron affinity N or P?
Solution : Electron affinity, in general decreases down a group. However, electron affinity of P(70) is more than N(0). It is due to the releatively compact 2p-subshell. Thus, electron affinity of P is more than N and As.
Which of the following has the highest electron affinity F Cl Br I?
Hence, chlorine has the highest electron affinity than fluorine and bromine.
See some more details on the topic Which of the following ion has highest electron affinity? here:
What Is Electron Affinity? | Trends & Chart | ChemTalk
Chlorine has the highest electron affinity among the elements. Its high affinity can be attributed to its large atomic radius, or size. Because …
Electron Affinity – The Periodic Table Variations Of Chemical …
Chlorine has the highest electron affinity while mercury has the lowest. Variation with Group and Row. Electron affinity generally increases across a period …
Periodic Trends — Electron Affinity
The halogens in Group 7A all have large negative electron affinities, since they are only one electron away from having a noble gas configuration, …
Electron Affinity | Introduction to Chemistry
The electron affinity of an atom or molecule is the propensity for that … added to it to form a negative ion, as demonstrated by the following equation:.
Which element has the highest electron affinity in period 2?
Fluorine is the element which has high electron affinity.
Which of the following species has the highest electron affinity?
Images related to the topicWhich of the following species has the highest electron affinity?

Does fluorine have the highest electron affinity?
Fluorine, which is higher up the group then chlorine, has a lower electron affinity. This is because the electrons in the outermost shell of a fluorine atom are closer together.
Which has higher electron affinity fluorine or neon?
Thus, noble gases have the least electron affinity in a period. Hence, we can conclude that in a period, fluorine (halogen) has higher electron affinity than neon (noble gas).
Which of the following has the highest electron affinity 1 F (-) 2 O (-) 3 O 4 Na?
Fluorine has highest electron affinity in the periodic table.
Which has the highest first electron affinity on SI?
Among all the given options, F atom has the highest electron affinity. (But, Cl has the highest electron affinity in the periodic table, F being the second highest). 19.
Which is bigger Cl or F?
Cl is somewhat larger than F. Cl and F are both members of the same family (Group-17). The main quantum number (n) of halogens grows as we proceed down the group, and the valence electrons migrate further away from the nucleus.
What is the order of electron affinity?
Electron affinity is the tendency is attract shared pair of electron towards itself. Its value decreases in a group and increases in a period. The correct order of electron affinities is as follows N<O<S<Cl.
Which has higher electron affinity carbon or Sulphur?
Electronegativity is the tendency of an atom to attract shared pair of electrons towards itself. Electronegativity increases from left to right in a period while it decreases down the group. Hence, Carbon is more electronegative than Sulphur because C lies in second period while Sulphur lies in third period.
Electron Affinity Trend, Basic Introduction, Chemistry
Images related to the topicElectron Affinity Trend, Basic Introduction, Chemistry
Which of the following is electron affinity?
The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. X ( g ) + e − → X − ( g ) + E .
Which of the following have highest electron affinity A NB OC Fd Cl?
Correct option d Cl Explanation:Due to smallest atomic sizes amongst the elements in their respective groups the elements of second period have lower electron affmities loweithan the corresponding elements of the third period. So chlorine has highest electron affinity.
Related searches to Which of the following ion has highest electron affinity?
- which of the following ion has highest electron affinity *
- periodic trends in electron affinity
- which of the following ion has highest electron affinity and electronegativity
- highest electron affinity is shown by
- electron affinity of elements
- Periodic trends in electron affinity
- Positive electron affinity
- Electron affinity table
- electron affinity
- why electron affinity decreases down the group
- which of the following ion has highest electron affinity in the periodic table
- Electron affinity of elements
- positive electron affinity
- Electron affinity
- the have the most negative electron affinities
- electron affinity table
- which of the following ion has highest electron affinity (most negative value)
Information related to the topic Which of the following ion has highest electron affinity?
Here are the search results of the thread Which of the following ion has highest electron affinity? from Bing. You can read more if you want.
You have just come across an article on the topic Which of the following ion has highest electron affinity?. If you found this article useful, please share it. Thank you very much.
