Are you looking for an answer to the topic “Which type of reaction is electrolysis of water?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Explanation: The electrolysis of water is an example of a non spontaneous redox reaction that occurs in the presence of electricity (energy).In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell.Electrolysis of water to form oxygen and hydrogen is an endothermic reaction because electrical energy is absorbed during this reaction.
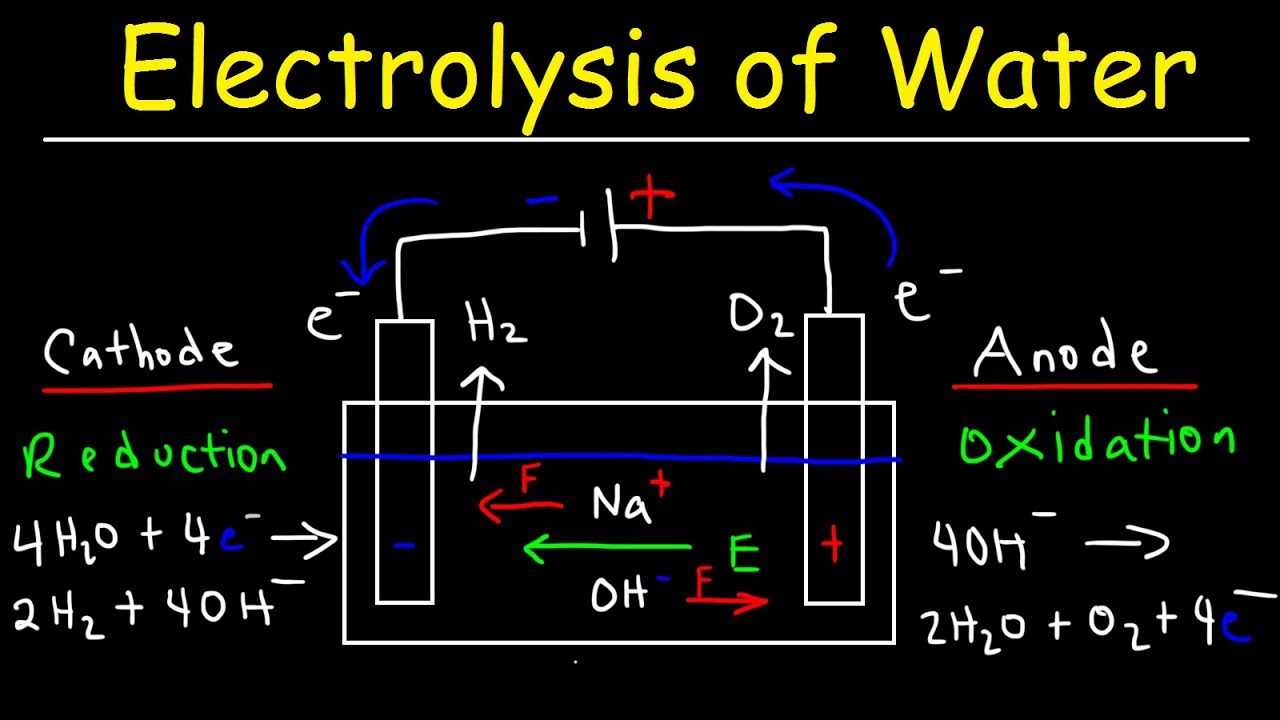
Which type of reaction is electrolysis?
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell.
Is electrolysis of water an endothermic reaction?
Electrolysis of water to form oxygen and hydrogen is an endothermic reaction because electrical energy is absorbed during this reaction.
Electrolysis of Water – Electrochemistry
Images related to the topicElectrolysis of Water – Electrochemistry

Is electrolysis of water a combination reaction?
Electrolysis of water is a decomposition reaction.
Is electrolysis of water a double displacement reaction?
Electrolysis of water is an example of this reaction. Two compounds exchange ions to form new compounds. Only a single product is formed. Reaction between MgO and water is an example for this reaction.
Is electrolysis a chemical reaction?
electrolysis, process by which electric current is passed through a substance to effect a chemical change. The chemical change is one in which the substance loses or gains an electron (oxidation or reduction).
Why is electrolysis of water is an exothermic reaction?
EXPLANATION: An endothermic reaction is the one that absorbs or uses heat from its surroundings. Water for electrolysis needs heat i.e. heat is absorbed and not given out which makes electrolysis an endothermic reaction. Endothermic reaction can be a chemical process or a physical process.
Is electrolysis endo or exothermic?
Some examples of endothermic reactions are: electrolysis.
See some more details on the topic Which type of reaction is electrolysis of water? here:
Electrolysis of water is which type of reaction? – Toppr
Electrolysis of water is which type of reaction? · Endothermic · Decomposition · Combination · Both (1) and (2) · Electrolysis of water is the decomposition of water …
Electrolysis of water – Wikipedia
Electrolysis of water, also known as electrochemical water splitting, is the process of using electricity to decompose water into oxygen and hydrogen gas by …
23.9: Electrolysis of Water – Chemistry LibreTexts
The electrolysis of water produces hydrogen and oxygen gases. … ion and hydroxide ions produced in each reaction combine to form water.
Electrolysis of Water – Energy Foundations for High School …
Summary. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen …
Is electrolysis a spontaneous reaction?
Explanation: Electrolytic cells use non-spontaneous reactions that require an external power source in order to proceed. The values between galvanic and electrolytic cells are opposite of one another. Galvanic cells have positive voltage potentials, while electrolytic voltage potentials are negative.
Electrolysis of Water Hydrochloric Acid | Reactions | Chemistry | FuseSchool
Images related to the topicElectrolysis of Water Hydrochloric Acid | Reactions | Chemistry | FuseSchool

What type of chemical reaction takes place when electricity is passed through acidulated water?
Explanation: When an electric current is passed through acidulated water, it will produce electrolysis. During this process, water is decomposed into hydrogen and oxygen.
Is electrolysis of water thermal decomposition?
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen (H2) due to an electric current being passed through the water.
Which among the following is double displacement reaction?
Double displacement reactions are those in which ions of the reactants are exchanged to form new compounds as products. respectively. Sodium and Barium are displaced from each other’s salts hence it is a double displacement reaction.
What type of chemical reaction is involved in an electrochemical reaction?
Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known as an oxidation-reduction (“redox”) reaction.
What is the process of electrolysis?
Electrolysis is the process by which ionic substances are decomposed (broken down) into simpler substances when an electric current is passed through them. Electricity is the flow of electrons or ions. For electrolysis to work, the compound must contain ions.
Is a redox reaction a chemical reaction?
oxidation-reduction reaction, also called redox reaction, any chemical reaction in which the oxidation number of a participating chemical species changes.
Experiment/ Electrolysis of water (Decomposition reaction by Electricity)/Activity
Images related to the topicExperiment/ Electrolysis of water (Decomposition reaction by Electricity)/Activity

Which is not an endothermic reaction?
It means combustion is an exothermic reaction.
Is the synthesis of water exothermic or endothermic?
It is given to us that formation of water from hydrogen and oxygen gas is exothermic, which means the energy of the reactant is higher than that of the products.
Related searches to Which type of reaction is electrolysis of water?
- electrolysis of water is an example of which reaction
- is electrolysis of water a redox reaction
- Electrolysis of water
- aluminum reacts with oxygen gas producing solid aluminum oxide what type of reaction is this
- what type of reaction is electrolysis
- electrolysis of water
- the electrolysis of water into hydrogen and oxygen is an example of double replacement reaction
- electrolysis of water is which type of reaction exothermic or endothermic
- electrolysis of water reaction
- what reaction is electrolysis of water
- what kind of reaction is electrolysis of water
- electrolysis of water is an example of which type of reaction
- electrolysis of acidified water is which type of reaction
- the electrolysis of water into hydrogen and oxygen is an example of what chemical reaction
- electrolysis of water is which type of reaction class 10
- in the electrolysis of water, _______ is used to provide energy.
- electrolysis of water balanced equation
- what is the reaction of electrolysis of water
- which type of reaction is electrolysis of water
- in the electrolysis of water is used to provide energy
Information related to the topic Which type of reaction is electrolysis of water?
Here are the search results of the thread Which type of reaction is electrolysis of water? from Bing. You can read more if you want.
You have just come across an article on the topic Which type of reaction is electrolysis of water?. If you found this article useful, please share it. Thank you very much.