Are you looking for an answer to the topic “What is equilibrium temperature class 7?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Ans: Heat always from a body of higher temperature to a body of lower temperature, until the temperature of both bodies are equal, then the two bodies are said to be in thermal equilibrium.Similarly, Earth has an equilibrium temperature of 255 K (−18 °C; −1 °F), but a surface temperature of about 288 K due to the greenhouse effect in our lower atmosphere.Two systems are in thermal equilibrium if there is no transfer of heat between them and both have the same temperature i.e. in accordance with the zeroth law of thermodynamics.

What is equilibrium temperature?
Similarly, Earth has an equilibrium temperature of 255 K (−18 °C; −1 °F), but a surface temperature of about 288 K due to the greenhouse effect in our lower atmosphere.
What is meant by thermal equilibrium Class 7?
Two systems are in thermal equilibrium if there is no transfer of heat between them and both have the same temperature i.e. in accordance with the zeroth law of thermodynamics.
Class 7. Heat. Equilibrium temperature. Units of heat.
Images related to the topicClass 7. Heat. Equilibrium temperature. Units of heat.

What is thermal equilibrium short answer?
Definition of thermal equilibrium
: a state of a system in which all parts are at the same temperature.
What is thermal equilibrium Byjus?
To discuss temperature changes, two basic concepts are important: thermal contact and thermal equilibrium. Two objects are in thermal contact if they can affect each other’s temperature. Thermal equilibrium exists when two objects in thermal contact no longer affect each other’s temperature.
What is equilibrium temperature formula?
ln (K1 / K2) = – (DH / R) (1/T1 – 1/T2). This is achieved by definite integral. This relationship indicates that the plot of ln (K versus 1/(T is a straight line, and the slope is – (DH / R). Thus, DH can be determined by measuring the equilibrium constant at different temperatures.
What is thermal equilibrium Ncert?
Heat will flow till both bodies acquire same temperature. This state when there is no heat flow between two bodies when they acquire the same temperature is known as thermal equilibrium.
What is thermal equilibrium shaala?
Thermal equilibrium exists when two objects in thermal contact no longer affect each other’s temperature (or) Both the objects are in the same temperature they are in thermal equilibrium.
See some more details on the topic What is equilibrium temperature class 7? here:
Thermal Equilibrium: Definition, Formula & Example – Study.com
Thermal equilibrium is a state of equivalent temperature between two objects whereby no energy is being exchanged, despite no impermeable …
What is thermal equilibrium example? – Byju’s
When an object or a body is brought in contact with another body with different temperatures, then the heat gets transferred from high temperature to low …
Heat and temperature (article) | Khan Academy
The zeroth law of thermodynamics says that no heat is transferred between two objects in thermal equilibrium; therefore, they are the same temperature.
2. Temperature and Thermal Equilibrium – BC Open Textbooks
Thermal equilibrium occurs when two bodies are in contact with each other and can freely exchange energy. Systems are in thermal equilibrium when they have the …
What is thermal equilibrium give one example of equilibrium state and explain?
The zeroth law of thermodynamics uses thermal equilibrium to define how two different systems can be said to be at the same temperature. For example, when molten rock comes up from a volcano, it will give off heat to the atmosphere until the rock and the atmosphere are at the same temperature.
What is thermal equilibrium diagram?
9.12 The thermal-equilibrium diagram is in reality a chart which shows the relationship between the composition, temperature and structure of any alloy in a series.
Thermal Equlibrium
Images related to the topicThermal Equlibrium
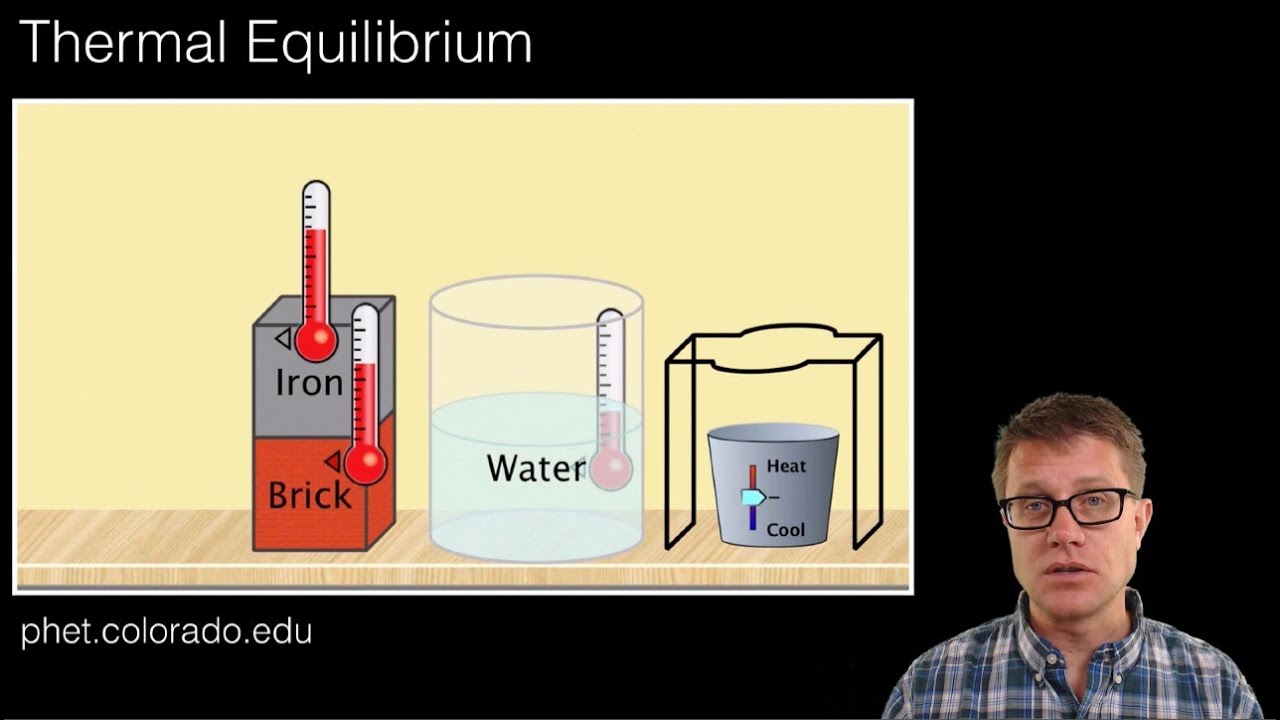
Why is there a thermal equilibrium?
Thermal equilibrium occurs when two bodies are in contact with each other and can freely exchange energy. Systems are in thermal equilibrium when they have the same temperature.
What is equilibrium chemistry?
chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and products occurs. A reversible chemical reaction is one in which the products, as soon as they are formed, react to produce the original reactants.
What is thermal equilibrium in 10th class?
When an object or a body is brought in contact with another body with different temperatures, then the heat gets transferred from high temperature to low temperature, till both the bodies attain thermal equilibrium.
What is thermal equilibrium Class 12?
Solution. Two systems are said to be in thermal equilibrium with each other if they are at the same temperature, which will not change with time.
What do you mean by equilibrium Class 11?
• Chemical Equilibrium. In a chemical reaction chemical equilibrium is defined as the state at which there is no further change in concentration of reactants and products. For example, At equilibrium the rate of forward reaction is equal to the rate of backward reaction.
What is an example of equilibrium?
An example of equilibrium is in economics when supply and demand are equal. An example of equilibrium is when you are calm and steady. An example of equilibrium is when hot air and cold air are entering the room at the same time so that the overall temperature of the room does not change at all.
What happens to equilibrium when temperature is increased?
if the temperature is increased, the position of equilibrium moves in the direction of the endothermic reaction. if the temperature is reduced, the position of equilibrium moves in the direction of the exothermic reaction.
What is the relationship between the equilibrium temperature and the greenhouse gas concentrations?
The ultimate increase in global average temperature corresponding to a given increase in greenhouse gas concentration is called the equilibrium global average temperature. Figure 3.4 shows possible impacts on the global equilibrium temperature of changes in atmospheric concentrations of greenhouse gases.
7.1 Le Chatelier’s principle (temperature) SL
Images related to the topic7.1 Le Chatelier’s principle (temperature) SL

What is thermodynamic equilibrium in physics?
thermodynamic equilibrium, condition or state of a thermodynamic system, the properties of which do not change with time and that can be changed to another condition only at the expense of effects on other systems.
What is temperature in 11th class?
Temperature is defined as the measure of degree of hotness or coldness of a body. A cup of hotsoup or a scoop of Ice-cream.
Related searches to What is equilibrium temperature class 7?
- what is heat
- what is the equilibrium temperature
- what is equilibrium temperature class 7
- systems in thermal equilibrium are at the same temperature is
- define temperature class 7
- definition of temperature for class 7
- how do you find the equilibrium temperature
- thermal equilibrium formula
- what is meant by equilibrium temperature
- what is temperature in physics class 7
- when two systems are in thermal equilibrium with a third system
- what is thermal equilibrium class 11
- explain thermal equilibrium with an example
- briefly explain the concept of thermal equilibrium and temperature
Information related to the topic What is equilibrium temperature class 7?
Here are the search results of the thread What is equilibrium temperature class 7? from Bing. You can read more if you want.
You have just come across an article on the topic What is equilibrium temperature class 7?. If you found this article useful, please share it. Thank you very much.