Are you looking for an answer to the topic “What Will Be Formed After Oxidation Reaction Of 2 Degree Alcohol With Chromic Acid?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Chromic acid is most commonly used to oxidize 2o alcohols to ketones.The chromic acid oxidizes secondary alcohol to a ketone. As shown in the below example it oxidizes isopropyl alcohol to acetone. Therefore, the correct option for the above answer is – option (D) ie Ketone.The oxidation of alcohols is an important reaction in organic chemistry. Primary alcohols can be oxidized to form aldehydes and carboxylic acids; secondary alcohols can be oxidized to give ketones. Tertiary alcohols, in contrast, cannot be oxidized without breaking the molecule’s C–C bonds.

What is formed after oxidation of 2 alcohol with chromic acid?
The chromic acid oxidizes secondary alcohol to a ketone. As shown in the below example it oxidizes isopropyl alcohol to acetone. Therefore, the correct option for the above answer is – option (D) ie Ketone.
What happened when two degree alcohol oxidize?
The oxidation of alcohols is an important reaction in organic chemistry. Primary alcohols can be oxidized to form aldehydes and carboxylic acids; secondary alcohols can be oxidized to give ketones. Tertiary alcohols, in contrast, cannot be oxidized without breaking the molecule’s C–C bonds.
Chromic acid oxidation of alcohols
Images related to the topicChromic acid oxidation of alcohols

Can you oxidize a secondary alcohol with chromic acid?
Alcohol Oxidation Reactions with Chromic Acid
Chromic Acid is the stronger of the two oxidizing agents. It will carry out both steps of oxidation of a primary alcohol producing a carboxylic acid as well as oxidizing secondary alcohols to ketones. It will also oxidize an aldehyde to a carboxylic acid.
What does chromic acid do to secondary alcohols?
Chromic acid oxidizes primary alcohols to carboxylic acids, and it oxidizes secondary alcohols to ketones.
What results are expected when the chromic acid test is done on primary alcohol?
The reaction of the Jonas reactant with a primary alcohol is given by the equation: A change in the solution’s color from red orange (chromic acid) to blue green (Cr(III)) ion indicates a positive result.
What is the formula of chromic acid?
What is formed after oxidation of 20 alcohol with chromic acid?
Chromic acid (H2CrO4) oxidizes alcohols in aqueous solutions of sodium dichromate. It reacts with alcohols to form a chromic ester in which the alcohol oxygen atom bridges the carbon and chromium atoms.
See some more details on the topic What Will Be Formed After Oxidation Reaction Of 2 Degree Alcohol With Chromic Acid? here:
Reactions of alcohols – Encyclopedia Britannica
Alcohols may be oxidized to give ketones, aldehydes, and carboxylic acids. … Tertiary alcohols do not react with chromic acid under mild conditions.
The Oxidation of Alcohols – ChemistryViews
The oxidation of alcohols is an important reaction in organic chemistry. Primary alcohols can be oxidized to form aldehydes and carboxylic …
19.6. Oxidation of alcohols & aldehydes | Organic Chemistry II
A common method for oxidizing secondary alcohols to ketones uses chromic acid (H2CrO4) as the oxidizing agent. Chromic acid, also known as Jones reagent, is …
4. Oxidation Reactions of Alcohols – MSU chemistry
The most generally useful reagents for oxidizing 1º and 2º-alcohols are chromic acid derivatives. Two such oxidants are Jones reagent (a solution of sodium …
Which of the following alcohols is oxidized to a ketone by chromic acid?
Chromic acid oxidizes primary alcohols to carboxylic acids, and it oxidizes secondary alcohols to ketones. Tertiary alcohols do not react with chromic acid under mild conditions.
How do you turn alcohol into an aldehyde?
The primary alcohol is converted to aldehyde by the oxidation reaction using mild oxidizing reagent.
What does chromic acid oxidize?
Chromic acid, H2CrO4, is a strong acid and a reagent for oxidizing alcohols to ketones and carboxylic acids.
Which alcohol will not be oxidized by chromic acid?
Tertiary alcohols do not react with chromic acid under mild conditions. With a higher temperature or a more concentrated, carbon-carbon bonds may be oxidized; however, yields from such strong oxidations are usually poor.
Alcohol Oxidation Mechanism with H2CrO4, PCC and KMnO4
Images related to the topicAlcohol Oxidation Mechanism with H2CrO4, PCC and KMnO4
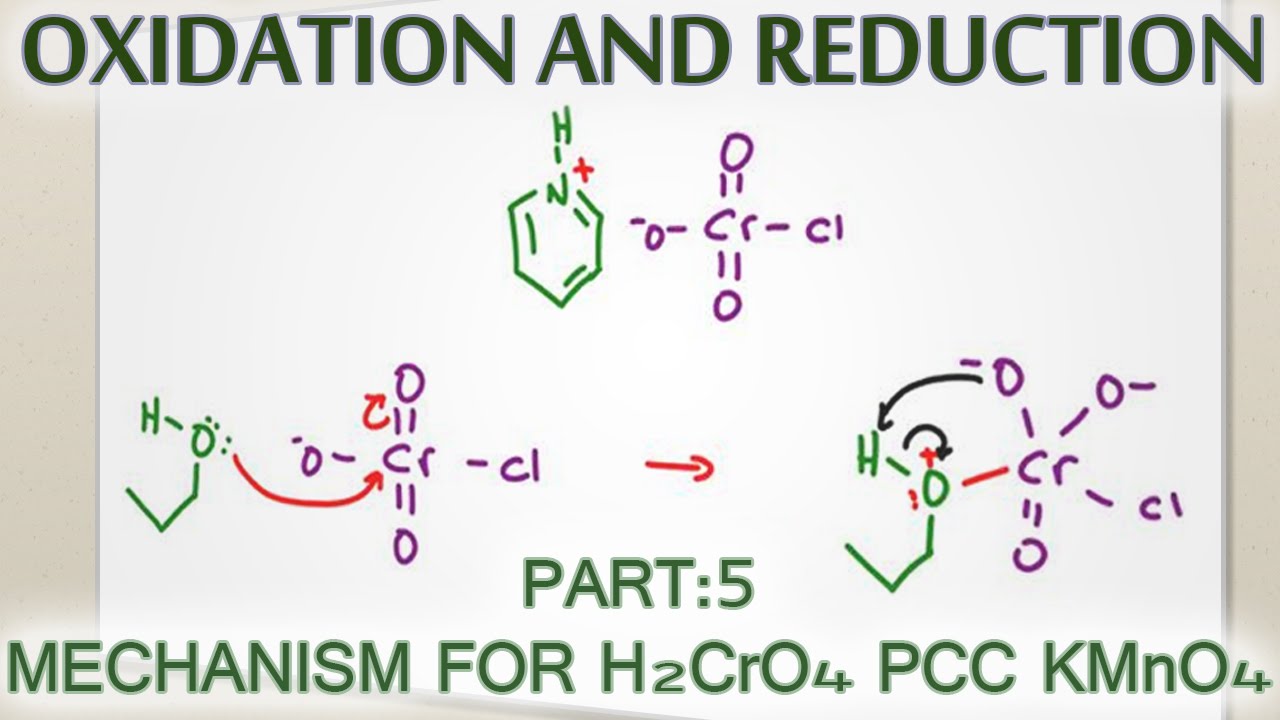
Is chromic acid an ideal oxidizing agent?
Chromic acid is a strong oxidizing agent, used to oxidize many classes of organic compounds, the most common of which is alcohols.
Which product is obtained by oxidation of phenol with chromic acid?
In the question, phenol is given as the starting material, but in the options, the product is either dione or diol. The product suggests that the starting material is hydroquinone. So instead of phenol, you start the reaction with hydroquinone. In presence of chromic acid, hydroquinone is oxidized to benzoquinone.
What gives a positive result in the chromic acid test?
Three drops of the compound to be tested are mixed with 5 drops of acetone and 5 drops of chromic acid solution (an orange solution). Indications of a positive test: The disappearance of the red-orange color of chromic acid and the formation of a blue-green color of the Cr (III) ion indicates a positive test.
How do you turn an alcohol into an alkene?
…
Dehydration of Alcohols to Yield Alkenes
- 1° alcohols: 170° – 180°C.
- 2° alcohols: 100°– 140 °C.
- 3° alcohols: 25°– 80°C.
How will you distinguish primary secondary and tertiary alcohol by Lucas test?
Solution : Lucas test is used to differentiate between primary, secondary and tertiary alcohol. Lucas reagent consists of equimolar mixture of con. HCl and anhydrous `ZnCl_2` <br> If turbidity appears immediately alcohols is tertiary. If turbidity appears in about five minutes the alcohols is secondary.
What kind of alcohol gives positive chromic acid test?
Test 1: Chromic Acid Oxidation
This test distinguishes primary and secondary alcohols from tertiary. Chromic acid will oxidize a primary alcohol first to an aldehyde and then to a carboxylic acid and it will oxidize a secondary alcohol to a ketone. Tertiary alcohols do not react.
How does chromic acid distinguish between an aldehyde and a ketone?
Aldehydes react with chromic acid gives a green to blue precipitate. Ketones do not react with chromic acid.
What is oxidation number of Cr in chromic acid?
Chromium forms several commercially valuable oxygen compounds, the most important of which is chromium oxide, commonly called chromium trioxide or chromic acid, CrO3, in which chromium is in the +6 oxidation state.
What is chromic acid solution?
Chromic acid is a commonly used glassware cleaning reagent. It is prepared in a one liter container by dissolving 60 grams of potassium dichromate in approximately 150 mls of warm distilled water and then slowly adding concentrated sulfuric acid to produce a total volume of one liter Chromic Acid solution.
Which is called chromic acid?
CrO3 is generally called chromic acid. Chromium trioxide CrO3 is the acidic anhydride of chromic acid. This is a dark-purple solid under anhydrous conditions, bright orange when wet and which dissolves in water concomitant with hydrolysis.
12.7 Oxidation with Chromic Acid and PCC
Images related to the topic12.7 Oxidation with Chromic Acid and PCC

What is the first oxidation product of secondary alcohol?
Ketone as it cannot be further oxidised easily.
Which one of the following compound is obtained by the Vigrous oxidation of tertiary alcohols?
An alcohol on vigorous oxidation is found to give ethanoic and propanoic acid.
Related searches to What Will Be Formed After Oxidation Reaction Of 2 Degree Alcohol With Chromic Acid?
- chromic acid test for alcohols
- oxidation of alcohol with k2cr2o7 mechanism
- oxidation of primary alcohol to aldehyde reagents
- oxidation of alcohol with kmno4
- chromic acid oxidation
- oxidation of tertiary alcohol with kmno4
- chromic acid test for aldehydes and ketones
- oxidation of primary alcohols
Information related to the topic What Will Be Formed After Oxidation Reaction Of 2 Degree Alcohol With Chromic Acid?
Here are the search results of the thread What Will Be Formed After Oxidation Reaction Of 2 Degree Alcohol With Chromic Acid? from Bing. You can read more if you want.
You have just come across an article on the topic What Will Be Formed After Oxidation Reaction Of 2 Degree Alcohol With Chromic Acid?. If you found this article useful, please share it. Thank you very much.